Abstract
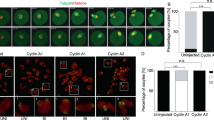
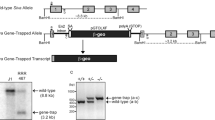
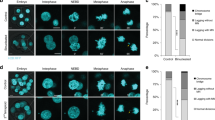
In higher eukaryotes, cell cycle progression is controlled by cyclin dependent kinases (Cdks) complexed with cyclins. A-type cyclins are involved at both G1/S and G2/M transitions of the cell cycle. Cyclin A2 activates cdc2 (Cdk1) on passage into mitosis and Cdk2 at the G1/S transition1. Antisense constructs, or antibodies directed against cyclin A2 block cultured mammalian cells at both of these transitions2–4. In contrast, overexpression of cyclin A2 appears to advance S phase entry5,6 and confer anchorage-independent growth7, and can lead to apoptosis8. A second A-type cyclin, cyclin A1 has been described recently which, in the mouse, is expressed in germ cells but not somatic tissues9,10. To address the possible redundancy between different cyclins in vivo and also the control of early embryonic cell cycles, we undertook the targeted deletion of the murine cyclin A2 gene. The homozygous null mutant is embryonically lethal, demonstrating that the cyclin A2 gene is essential. Surprisingly, homozygous null mutant embryos develop normally until post-implantation, around day 5.5 p.c. This observation may be explained by the persistence of a maternal pool of cyclin A2 protein until at least the blastocyst stage, or an unexpected role for cyclin A1 during early embryo development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nigg, E.A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays. 17, 471–480 (1995).
Pagano, M., Pepperkok, R., Verde, F., Ansorge, W. & Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBOJ. 11, 961–971 (1992).
Girard, P., Strausfeld, U., Fernandez, A. & Lamb, N. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 67, 1169–1179 (1991).
Zindy, F. et al. Cyclin A is required in S phase in normal epithelial cells. Biochem. Biophys. Res. Comm. 182, 1144–1154 (1992).
Rosenberg, A. et al. Overexpression of human cyclin A advances entry into S phase. Oncogene 10, 1501–1509 (1995).
Resnitzky, D., Hengst, L. & Reed, S. Cyclin A associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27kip1. Mol. Cell. Biol. 15, 4347–4352 (1995).
Guadagno, T.M., Ohtsubo, M., Roberts, J.M. & Assoain, R.K. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 262, 1572–1574 (1993).
Bortner, D.M. & Rosenberg, M.P. Overexpression of cyclin A in the mammary glands of transgenic mice results in the induction of nuclear abnormalities and increased apoptosis. Cell Growth Diff. 6, 1579–1589 (1995).
Howe, J.A., Howell, M., Hunt, T. & Newport, J.W. Identification of a developmental timer regulating the stability of embryonic cyclin A and a new somatic A-type cyclin at gastrulation. Genes.Dev. 9, 1164–1176 (1995).
Sweeney, C. et al. Cyclin A is expressed in germ cells in the mouse. Development. 122, 53–64 (1996).
Cappecchi, M.R. Altering the genome by homologous recombination. Science. 244, 1288–1292 (1989).
Hogan, B., Beddington, R., Costantini, F. & Lacy, E. Manipulating the Mouse Embryo. 2nd edn. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1994).
Theiler, K. The House Mouse.(Springer-Verlag, New York, 1989).
Vernet, M., Briand, P & Nicolas, J.-F. (eds Wassarman, P.M. & DePamphilis, M.L.) 434–451 (Academic Press, San Diego, California, 1993).
Strausfeld, U.P. et al. Both cyclin A and cyclin E have S-phase promoting (SPF) activity in Xenopus egg extracts. J. Cell Sci. 109, 1555–1563 (1996).
Flach, G., Johnson, M.H., Braude, P.R., Taylor, R.A. & Bolton, V.N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1, 681–686 (1982).
Bachvarova, R. & De Leon, V. Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev. Biol. 74, 1–8 (1980).
Sicinski, P. et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–630 (1995).
Fantl, V., Stamp, G., Andrews, A., Rosewell, I. & Dickson, C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9, 2364–2372 (1995).
Thomas, K.R. & Cappecchi, M.R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51, 503–512 (1987).
Kühn, R., Rajewski, K. & Muller, W. Generation and analysis of lnterleukin-4 deficient mice. Science 254, 707–710 (1991).
Bradley, A. (ed. Robertson, E.) 113–157 (IRL Press, Oxford, 1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murphy, M., Stinnakre, MG., Senamaud-Beaufort, C. et al. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet 15, 83–86 (1997). https://doi.org/10.1038/ng0197-83
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0197-83
This article is cited by
-
Challenges and advances in clinical applications of mesenchymal stromal cells
Journal of Hematology & Oncology (2021)
-
Association of ESX1 gene variants with non-obstructive azoospermia in Chinese males
Scientific Reports (2021)
-
Multiomics global landscape of stemness-related gene clusters in adipose-derived mesenchymal stem cells
Stem Cell Research & Therapy (2020)
-
Essential role of the Cdk2 activator RingoA in meiotic telomere tethering to the nuclear envelope
Nature Communications (2016)
-
To be or not to be a proliferation marker?
Oncogene (2014)