Abstract
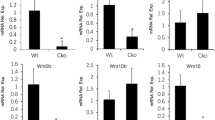
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGF-β) superfamily. Many BMPs are produced in bone and show osteogenic activity, suggesting that they may be determinants of bone mass. BMP3 was originally purified from bone as osteogenin, which induces osteogenic differentiation1. Recombinant BMP3 (rhBMP3) has no biological activity, however, leaving its role in skeletal growth unclear. Here we show that BMP3 is an antagonist of osteogenic BMPs: BMP3 dorsalizes Xenopus laevis embryos, inhibits BMP2-mediated induction of Msx2 and blocks BMP2-mediated differentiation of osteoprogenitor cells into osteoblasts. These effects appear to be mediated through activin receptors. Finally, Bmp3−/− mice have twice as much trabecular bone as wild-type littermates, indicating that BMP3, the most abundant BMP in adult bone, is a negative determinant of bone density.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Luyten, F.P., Yanagishita, M., Vukicevic, S., Hammonds, R.G. & Reddi, A.H. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J. Biol. Chem. 267, 3691–3695 (1992).
Luyten, F.P. et al. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J. Biol. Chem. 264, 13377–13380 (1989).
Wozney, J.M. & Rosen, V. in Physiology and Pharmacology of Bone (eds. Martin, T.J. & Mundy, G.) 725–748 (Springer-Verlag, Berlin, 1993)
Takao, M. et al. Identification of rat bone morphogenetic protein-3b (BMP3b), a new member of BMP-3. Biochem. Biophys. Res. Commun. 219, 656–662 (1996).
Thies, R.S. et al. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology 130, 1318–1324 (1992).
Engstrand, T. et al. Transient production of BMP-2 by allogeneic transplanted transduced cells induces bone formation. Hum. Gene Ther. 11, 205–211 (2000).
Harland, R. The transforming growth factor β family and induction of the vertebrate mesoderm: bone morphogenetic proteins are ventral inducers. Proc. Natl. Acad. Sci. USA 91, 10243–10246 (1994).
Hazama, M., Aona, A., Ueno, N. & Fujisawa, Y. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem. Biophys. Res. Commun. 209, 859–866 (1995).
Katagiri, T. et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127, 1755–1766 (1994).
Hollnagel, A. et al. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem. 274, 19838–19845 (1999).
Liu, Y.H. et al. Premature suture closure and ectopic cranial bone in mice expressing Msx-2 transgenes in the developing skull. Proc. Natl. Acad. Sci. USA 92, 6137–6141 (1995).
Brummel, T.J. et al. Characterization and relationship of dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell 78, 251–261 (1994).
Penton, A. et al. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell 78, 239–250 (1994).
Hoodless, P.A. et al. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell 85, 489–500 (1996).
Attisano, L. et al. Identification of human activin and TGF-β type I receptors that form hetermeric kinase complexes with type II receptors. Cell 75, 671–680 (1993).
Macías-Silva, M. et al. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 273, 25628–25636 (1998).
Zhao, R., Lawler, A.M. & Lee, S.-J. Characterization of GDF-10 expression patterns and null mice. Dev. Biol. 212, 68–79 (1999).
Filvaroff, E. et al. Inhibition of TGF-β receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development 126, 4267–4279 (1999).
Li, B. et al. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nature Genet. 24, 304–308 (2000).
Smith, W.C. TGF β inhibitors. New and unexpected requirements in vertebrate development. Trends Genet. 15, 3–5 (1999).
Piek, E. et al. Functional antagonism between activin and osteogenic protein-1 in human embryonal carcinoma cells. J. Cell Physiol. 180, 141–149 (1999).
Candia, A.F. et al. Cellular interpretation of multiple TGF-β signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 124, 4467–4480 (1997).
Ikenoue, T. et al. Inhibitory effects of activin-A on osteoblast differentiation during cultures of fetal rat calvarial cells. J. Cell Biochem. 75, 206–214 (1999).
Centrella, M., McCarthy, T.L. & Canalis, E. Activin-A binding and biochemical effects in osteoblast-enriched cultures from fetal-rat parietal bone. Mol. Cell. Biol. 11, 250–258 (1991).
Erlebacher, A. & Derynck, R. Increased expression of TGF-β2 in osteoblasts results in an osteoporosis-like phenotype. J. Cell Biol. 132, 195–210 (1996).
Centrella, M. et al. Independent changes in type I and type II receptors for transforming growth factor β by bone morphogenetic protein 2 parallel expression of the osteoblast phenotype. Mol. Cell. Biol. 15, 3273–3281 (1995).
Zmuda, J.M., Cauley, J.A. & Ferrell, R.E. Recent progress in understanding the genetic susceptibility to osteoporosis. Genet. Epidemiol. 16, 356–367 (1999).
Bostrom, M.P.G. et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J. Orthop. Res. 13, 357–367 (1993).
Yi, S.E. et al. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127, 621–630 (2000).
Parfitt, A.M. et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. J. Bone Miner. Res. 2, 595–608 (1987).
Acknowledgements
We thank E. DeRobertis for assistance with X. laevis embryo injections; W. Goodman for guidance with histomorphometric analysis; D. Israel and J. Nove for generation of Bmp3-targeted ES cells; R. Peterson for help with initial breeding experiments; R. Maxson for 2 kb Msx2-Lux; K. Arora for CA-TKV; D. Litwack for rat Bmp3 cDNA; J. Massagué for ALK-4 and ActRII expression vectors; and J. Lengyel, U. Banerjee, A. Lusis and L. Birnbaumer for comments. This work was supported by NIH grant AR44528 (K.M.L.) and the Wendy Will Case Cancer Fund (K.M.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daluiski, A., Engstrand, T., Bahamonde, M. et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 27, 84–88 (2001). https://doi.org/10.1038/83810
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83810
This article is cited by
-
The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease
Cell Research (2024)
-
Role of PDGFRA+ cells and a CD55+ PDGFRALo fraction in the gastric mesenchymal niche
Nature Communications (2023)
-
BMP3 is a novel locus involved in the causality of ocular coloboma
Human Genetics (2022)
-
Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria
Maxillofacial Plastic and Reconstructive Surgery (2021)
-
Non-union bone fractures
Nature Reviews Disease Primers (2021)