Abstract
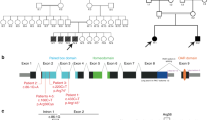
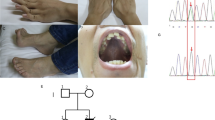
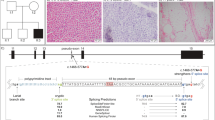
Infantile myopathies with diaphragmatic paralysis are genetically heterogeneous, and clinical symptoms do not assist in differentiating between them. We used phased haplotype analysis with subsequent targeted exome sequencing to identify MEGF10 mutations in a previously unidentified type of infantile myopathy with diaphragmatic weakness, areflexia, respiratory distress and dysphagia. MEGF10 is highly expressed in activated satellite cells and regulates their proliferation as well as their differentiation and fusion into multinucleated myofibers, which are greatly reduced in muscle from individuals with early onset myopathy, areflexia, respiratory distress and dysphagia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Accession codes
References
Grohmann, K. et al. Nat. Genet. 29, 75–77 (2001).
Guenther, U.P. et al. J. Mol. Med. 87, 31–41 (2009).
Guenther, U.P. et al. Hum. Mutat. 28, 808–815 (2007).
Grohmann, K. et al. Hum. Mol. Genet. 13, 2031–2042 (2004).
Maddatu, T.P. et al. Hum. Mol. Genet. 13, 1105–1115 (2004).
Pitt, M. et al. Brain 126, 2682–2692 (2003).
Hartley, L. et al. Neuromuscul. Disord. 17, 174–179 (2007).
Carr, I.M. et al. Hum. Mutat. 30, 960–967 (2009).
Seelow, D. et al. Nucleic Acids Res. 37, W593–W599 (2009).
Suzuki, E. et al. Exp. Cell Res. 313, 3729–3742 (2007).
Suzuki, E. et al. Exp. Cell Res. 313, 2451–2464 (2007).
Hamon, Y. et al. PLoS ONE 1, e120 (2006).
Wu, H.H. et al. Nat. Neurosci. 12, 1534–1541 (2009).
Nagase, T. et al. DNA Res. 8, 85–95 (2001).
Holterman, C.E. et al. J. Cell Biol. 179, 911–922 (2007).
Acknowledgements
The authors thank the families who participated in this study. F.M. is supported by the Great Ormond Street Hospital Children's Charity. Z.A.A. receives an Egyptian Government Scholarship. This work was supported by grants from Newlife Foundation for Disabled Children (to I.M.C. and C.A.J.) and the Sir Jules Thorn Award for Biomedical Research (to C.A.J., E.S., G.R.T. and D.T.B.), the Deutsche Forschungsgemeinschaft (SFB 665 TP C4 and KFO 192) and the NeuroCure Cluster of Excellence, Exc 257 (to M. Schuelke) and the parents' support group 'Helft dem muskelkranken Kind' Hamburg, Germany (to C.H.). We acknowledge the contribution of clinical data by U. Schara and T. Polster.
Author information
Authors and Affiliations
Contributions
K.S., M. Schuelke and I.M.C. performed genetic mapping. C.V.L., B.L., D.A.P., C.P.D., G.M., M. Schuelke and C.A.J. performed mutation analyses in the cohorts of affected individuals. C.V.L., J.E.M., D.A.P. and G.R.T. generated the next-generation sequencing data. C.V.L. and D.A.P. performed the control genotyping. D.A.P., I.M.C., M. Schuelke and G.R.T. analyzed the SNP genotyping and next-generation sequencing data. C.V.L. examined the complementary DNA and protein expression in cell lines from affected individuals. Z.A.A. and M. Shires performed the immunohistochemistry staining experiments. C.P., A.v.R., I.E., A.F.M., T.H., E.S., C.H., F.M., A.Y.M., M. Scoto and M. Schuelke recruited subjects, gathered clinical information and contributed clinical samples. A.F.M., D.T.B., E.S., F.M., I.M.C., C.H., M. Schuelke and C.A.J. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1 and 2, Supplementary Methods and Supplementary Note. (PDF 2074 kb)
Rights and permissions
About this article
Cite this article
Logan, C., Lucke, B., Pottinger, C. et al. Mutations in MEGF10, a regulator of satellite cell myogenesis, cause early onset myopathy, areflexia, respiratory distress and dysphagia (EMARDD). Nat Genet 43, 1189–1192 (2011). https://doi.org/10.1038/ng.995
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.995
This article is cited by
-
The Notch signaling network in muscle stem cells during development, homeostasis, and disease
Skeletal Muscle (2022)
-
Exploiting genomic synteny in Felidae: cross-species genome alignments and SNV discovery can aid conservation management
BMC Genomics (2021)
-
Controlling T cells spreading, mechanics and activation by micropatterning
Scientific Reports (2021)
-
Congenital myopathies: disorders of excitation–contraction coupling and muscle contraction
Nature Reviews Neurology (2018)
-
Genomic signatures of local adaptation reveal source-sink dynamics in a high gene flow fish species
Scientific Reports (2017)