Abstract
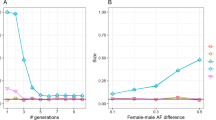
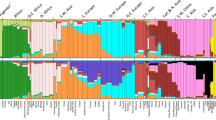
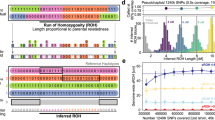
Studies of recombination and how it varies depend crucially on accurate recombination maps. We propose a new approach for constructing high-resolution maps of relative recombination rates based on the observation of ancestry switch points among admixed individuals. We show the utility of this approach using simulations and by applying it to SNP genotype data from a sample of 2,565 African Americans and 299 African Caribbeans and detecting several hundred thousand recombination events. Comparison of the inferred map with high-resolution maps from non-admixed populations provides evidence of fine-scale differentiation in recombination rates between populations. Overall, the admixed map is well predicted by the average proportion of admixture and the recombination rate estimates from the source populations. The exceptions to this are in areas surrounding known large chromosomal structural variants, specifically inversions. These results suggest that outside of structurally variable regions, admixture does not substantially disrupt the factors controlling recombination rates in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Crawford, D.C. et al. Evidence for substantial fine-scale variation in recombination rates across the human genome. Nat. Genet. 36, 700–706 (2004).
Evans, D.M. & Cardon, L. A comparison of linkage disequilibrium patterns and estimated population recombination rates across multiple populations. Am. J. Hum. Genet. 76, 681–687 (2005).
Graffelman, J., Balding, D., Gonzalez-Neira, A. & Bertranpetit, J. Variation in estimated recombination rates across human populations. Hum. Genet. 122, 301–310 (2007).
Serre, D., Nadon, R. & Hudson, T.J. Large-scale recombination rate patterns are conserved among human populations. Genome Res. 15, 1547–1552 (2005).
Laayouni, H. et al. Similarity in recombination rate estimates highly correlates with genetic differentiation in humans. PLoS ONE 6, e17913 (2011).
Clark, A.G., Wang, X. & Matise, T. Contrasting methods of quantifying fine structure of human recombination. Annu. Rev. Genomics Hum. Genet. 11, 45–64 (2010).
Kong, A. et al. A high-resolution recombination map of the human genome. Nat. Genet. 31, 241–247 (2002).
Kong, A. et al. Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467, 1099–1103 (2010).
Broman, K.W., Murray, J.C., Sheffield, V.C., White, R.L. & Weber, J.L. Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am. J. Hum. Genet. 63, 861–869 (1998).
Coop, G., Wen, X., Ober, C., Pritchard, J.K. & Przeworski, M. High-resolution mapping of crossovers reveals extensive variation in fine-scale recombination patterns among humans. Science 319, 1395–1398 (2008).
Jorgenson, E. et al. Ethnicity and human genetic linkage maps. Am. J. Hum. Genet. 76, 276–290 (2005).
Ju, Y.S. et al. A genome-wide Asian genetic map and ethnic comparison: the GENDISCAN study. BMC Genomics 9, 554 (2008).
International HapMap Consortium. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Myers, S., Bottolo, L., Freeman, C., McVean, G. & Donnelly, P.A. Fine-scale map of recombination rates and hotspots across the human genome. Science 310, 321–324 (2005).
O'Reilly, P.F., Birney, E. & Balding, D.J. Confounding between recombination and selection, and the Ped/Pop method for detecting selection. Genome Res. 18, 1304–1313 (2008).
McVean, G.A.T. et al. The fine-scale structure of recombination rate variation in the human genome. Science 304, 581–584 (2004).
Price, A.L. et al. Sensitive detection of chromosomal segments of distinct ancestry in admixed populations. PLoS Genet. 5, e1000519 (2009).
Johnson, A.D. et al. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat. Genet. 42, 608–613 (2010).
Daniels, P.R. et al. Familial aggregation of hypertension treatment and control in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Am. J. Med. 116, 676–681 (2004).
FBPP Investigators. Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP). Hypertension 39, 3–9 (2002).
Barnes, K.C. et al. Linkage of asthma and total serum IgE concentration to markers on chromosome 12q: evidence from Afro-Caribbean and Caucasian populations. Genomics 37, 41–50 (1996).
Mathias, R.A. et al. A genome-wide association study on African-ancestry populations for asthma. J. Allergy Clin. Immunol. 125, 336–346.e4 (2010).
Zambelli-Weiner, A. et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J. Allergy Clin. Immunol. 115, 1203–1209 (2005).
Moore, W.C. et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J. Allergy Clin. Immunol. 119, 405–413 (2007).
Bryc, K. et al. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc. Natl. Acad. Sci. USA 107, 786–791 (2010).
Murray, T. et al. African and non-African admixture components in African Americans and an African Caribbean population. Genet. Epidemiol. 34, 561–568 (2010).
Parra, E.J. et al. Estimating African American admixture proportions by use of population-specific alleles. Am. J. Hum. Genet. 63, 1839–1851 (1998).
Tang, H. et al. Recent genetic selection in the ancestral admixture of Puerto Ricans. Am. J. Hum. Genet. 81, 626–633 (2007).
Antonacci, F. et al. Characterization of six human disease-associated inversion polymorphisms. Hum. Mol. Genet. 18, 2555–2566 (2009).
Deng, L. et al. An unusual haplotype structure on human chromosome 8p23 derived from the inversion polymorphism. Hum. Mutat. 29, 1209–1216 (2008).
Giglio, S. et al. Olfactory receptor-gene clusters, genomic-inversion polymorphisms, and common chromosome rearrangements. Am. J. Hum. Genet. 68, 874–883 (2001).
Kidd, J.M. et al. Mapping and sequencing of structural variation from eight human genomes. Nature 453, 56–64 (2008).
Redon, R. et al. Global variation in copy number in the human genome. Nature 444, 444–454 (2006).
Stefansson, H. et al. A common inversion under selection in Europeans. Nat. Genet. 37, 129–137 (2005).
Bergström, T.F., Josefsson, A., Erlich, H.A. & Gyllensten, U. Recent origin of HLA-DRB1 alleles and implications for human evolution. Nat. Genet. 18, 237–242 (1998).
The 1,000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Berg, I.L. et al. PRDM9 variation strongly influences recombination hot-spot activity and meiotic instability in humans. Nat. Genet. 42, 859–863 (2010).
Baudat, F. et al. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327, 836–840 (2010).
Bansal, V., Bashir, A. & Bafna, V. Evidence for large inversion polymorphisms in the human genome from HapMap data. Genome Res. 17, 219–230 (2007).
Price, A.L. et al. Long-range LD can confound genome scans in admixed populations. Am. J. Hum. Genet. 83, 132–135, author reply 135–139 (2008).
Feuk, L. et al. Discovery of human inversion polymorphisms by comparative analysis of human and chimpanzee DNA sequence assemblies. PLoS Genet. 1, e56 (2005).
Conrad, D.F. et al. Origins and functional impact of copy number variation in the human genome. Nature 464, 704–712 (2010).
Itsara, A. et al. Population analysis of large copy number variants and hotspots of human genetic disease. Am. J. Hum. Genet. 84, 148–161 (2009).
Tuzun, E. et al. Fine-scale structural variation of the human genome. Nat. Genet. 37, 727–732 (2005).
McKernan, K.J. et al. Sequence and structural variation in a human genome uncovered by short-read, massively parallel ligation sequencing using two-base encoding. Genome Res. 19, 1527–1541 (2009).
Zogopoulos, G. et al. Germ-line DNA copy number variation frequencies in a large North American population. Hum. Genet. 122, 345–353 (2007).
de Smith, A.J. et al. Array CGH analysis of copy number variation identifies 1,284 new genes variant in healthy white males: implications for association studies of complex diseases. Hum. Mol. Genet. 16, 2783–2794 (2007).
Long, J.C. The genetic structure of admixed populations. Genetics 127, 417–428 (1991).
Pfaff, C.L. et al. Population structure in admixed populations: effect of admixture dynamics on the pattern of linkage disequilibrium. Am. J. Hum. Genet. 68, 198–207 (2001).
Pool, J.E. & Nielsen, R. Inference of historical changes in migration rate from the lengths of migrant tracts. Genetics 181, 711–719 (2009).
Chen, G.K., Marjoram, P. & Wall, J.D. Fast and flexible simulation of DNA sequence data. Genome Res. 19, 136–142 (2009).
Schaffner, S.F. et al. Calibrating a coalescent simulation of human genome sequence variation. Genome Res. 15, 1576–1583 (2005).
Howie, B.N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Acknowledgements
J.N., D.W. and K.R.V. were funded by a Searle Scholar Program award to J.N. N.B.F. was supported by US National Institutes of Health (NIH) grants R01HL087679 and RL1MH083268. The sample assembled is compiled from the larger efforts and the generous sharing of data from four major consortiums. For the GeneSTAR consortium (L.C.B., D.R.B., L.R.Y. and R.A.M.), support came from NIH grants HL072518 and M01-RR00052. For the CAG-CSGA consortium (D.A.M., D.G.T. and D.L.N.), support came from NIH grants U01 HL49596, R01 HL072414, R01 HL087665 and RC2 HL101651, and special thanks is given to C. Ober. For the GENOA samples (Y.V.S. and S.L.R.K.), support came from NIH grants HL087660 and HL100245, and special thanks is given to E. Boerwinkle. For the GRAAD consortium (K.C.B., N.R., I.R., T.H.B. and R.A.M.), support came from NIH grants HL087699, HL49612, AI50024, AI44840, HL075417, HL072433, AI41040, ES09606, HL072433 and RR03048, US Environmental Protection Agency grant 83213901, and National Institute of General Medical Sciences (NIGMS) grant S06GM08015, and special thanks are given to A.V. Grant, L. Gao, C. Vergara, Y.J. Tsai, P. Gao, M.C. Liu, P. Breysse, M.B. Bracken, J. Hoh, E.W. Pugh, A.F. Scott, G. Abecasis, T. Murray, T. Hand, M. Yang, M. Campbell, C. Foster, J.B. Hetmanski, R. Ashworth, C.M. Ongaco, K.N. Hetrick and K.F. Doheny. K.C.B. was supported in part by the Mary Beryl Patch Turnbull Scholar Program. R.A.M. was supported in part by the Mosaic Initiative Award from Johns Hopkins University. We thank C. Jaquish and the NHLBI STAMPEED program for their support of this collaboration. We also acknowledge G. Coop, A. di Rienzo, K. Lohmueller, and M. Przeworski for helpful discussions and comments on a draft of the manuscript.
Author information
Authors and Affiliations
Contributions
J.N. and N.B.F. conceived of the project, and D.W., J.N., N.B.F. and D.L.N. designed the analyses. D.G.T. and D.L.N. worked as part of the Chicago Asthma Genetics (CAG) and Collaborative Study on the Genetics of Asthma (CGSA) consortium to gather and prepare primary data for subsequent analysis. R.A.M., L.R.Y., L.C.B. and D.M.B. worked as part of the Genetic Study of Atherosclerosis Risk (GeneSTAR) Consortium to gather and prepare primary data for subsequent analysis. I.R., N.R., R.A.M., T.H.B. and K.C.B. worked as part of the Genetic Research on Asthma in the African Diaspora (GRAAD) consortium to gather and prepare primary data for subsequent analysis. Y.V.S., T.M. and S.L.R.K. worked as part of the Genetic Epidemiology Network of Arteriopathy (GENOA) consortium to gather and prepare primary data for subsequent analysis. D.G.T. and D.A.M. worked as part of the Severe Asthma Research Program (SARP) to gather and prepare primary data for subsequent analysis. D.W., D.E.K., K.R.V. and J.N. developed tools for the analysis and performed the analysis. D.W., N.B.F. and J.N. drafted the manuscript and revised it with D.E.K., K.R.V., R.A.M., D.L.N., L.R.Y., Y.V.S., L.C.B., N.R., I.R., T.H.B., S.L.R.K., D.A.M., K.C.B. and D.M.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Tables 1–4 and Supplementary Figures 1–14. (PDF 5058 kb)
Rights and permissions
About this article
Cite this article
Wegmann, D., Kessner, D., Veeramah, K. et al. Recombination rates in admixed individuals identified by ancestry-based inference. Nat Genet 43, 847–853 (2011). https://doi.org/10.1038/ng.894
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.894
This article is cited by
-
Fine human genetic map based on UK10K data set
Human Genetics (2022)
-
The bracteatus pineapple genome and domestication of clonally propagated crops
Nature Genetics (2019)
-
Relatedness in the post-genomic era: is it still useful?
Nature Reviews Genetics (2015)
-
Development of a dense SNP-based linkage map of an apple rootstock progeny using the Malus Infinium whole genome genotyping array
BMC Genomics (2012)
-
Recombination diversified
Nature Reviews Genetics (2011)