Abstract
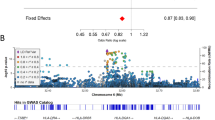
Parkinson's disease is a common disorder that leads to motor and cognitive disability. We performed a genome-wide association study of 2,000 individuals with Parkinson's disease (cases) and 1,986 unaffected controls from the NeuroGenetics Research Consortium (NGRC)1,2,3,4,5. We confirmed associations with SNCA2,6,7,8 and MAPT3,7,8,9, replicated an association with GAK9 (using data from the NGRC and a previous study9, P = 3.2 × 10−9) and detected a new association with the HLA region (using data from the NGRC only, P = 2.9 × 10−8), which replicated in two datasets (meta-analysis P = 1.9 × 10−10). The HLA association was uniform across all genetic and environmental risk strata and was strong in sporadic (P = 5.5 × 10−10) and late-onset (P = 2.4 × 10−8) disease. The association peak we found was at rs3129882, a noncoding variant in HLA-DRA. Two studies have previously suggested that rs3129882 influences expression of HLA-DR and HLA-DQ10,11. The brains of individuals with Parkinson's disease show upregulation of DR antigens and the presence of DR-positive reactive microglia12, and nonsteroidal anti-inflammatory drugs reduce Parkinson's disease risk4,13. The genetic association with HLA supports the involvement of the immune system in Parkinson's disease and offers new targets for drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Payami, H., Larsen, K., Bernard, S. & Nutt, J. Increased risk of Parkinson′s disease in parents and siblings of patients. Ann. Neurol. 36, 659–661 (1994).
Kay, D.M. et al. Genetic association between alpha-synuclein and idiopathic Parkinson's disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 147B, 1222–1230 (2008).
Zabetian, C.P. et al. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson′s disease. Ann. Neurol. 62, 137–144 (2007).
Powers, K.M. et al. Combined effects of smoking, coffee and NSAIDs on Parkinson′s disease risk. Mov. Disord. 23, 88–95 (2008).
McCulloch, C.C. et al. Exploring gene-environment interactions in Parkinson′s disease. Hum. Genet. 123, 257–265 (2008).
Maraganore, D.M. et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. J. Am. Med. Assoc. 296, 661–670 (2006).
Simón-Sánchez, J. et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 41, 1308–1312 (2009).
Edwards, T.L. et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet. 74, 97–109 (2010).
Pankratz, N. et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum. Genet. 124, 593–605 (2009).
Stranger, B.E. et al. Population genomics of human gene expression. Nat. Genet. 39, 1217–1224 (2007).
Montgomery, S.B. et al. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature 464, 773–777 (2010).
McGeer, P.L. & McGeer, E.G. Glial reactions in Parkinson′s disease. Mov. Disord. 23, 474–483 (2008).
Chen, H. et al. Nonsteroidal anti-inflammatory drug use and the risk for Parkinson′s disease. Ann. Neurol. 58, 963–967 (2005).
Ward, C.D. et al. Parkinson's disease in 65 pairs of twins and in a set of quadruplets. Neurology 33, 815–824 (1983).
Tanner, C.M. et al. Parkinson disease in twins: an etiologic study. J. Am. Med. Assoc. 281, 341–346 (1999).
Thacker, E.L. & Ascherio, A. Familial aggregation of Parkinson′s disease: a meta-analysis. Mov. Disord. 23, 1174–1183 (2008).
Mata, I.F. et al. A SNCA variant associated with Parkinson's disease and plasma α-synuclein level. Arch. Neurol. (in the press).
Hernán, M.A., Takkouche, B., Caamano-Isorna, F. & Gestal-Otero, J.J. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann. Neurol. 52, 276–284 (2002).
Satake, W. et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 41, 1303–1307 (2009).
Fung, H.C. et al. Genome-wide genotyping in Parkinson′s disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 5, 911–916 (2006).
Maraganore, D.M. et al. High-resolution whole-genome association study of Parkinson disease. Am. J. Hum. Genet. 77, 685–693 (2005).
Hamza, T.H. & Payami, H. The heritability of risk and age at onset of Parkinson′s disease after accounting for known genetic risk factors. J. Hum. Genet. 55, 241–243 (2010).
Gibb, W.R. & Lees, A. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 51, 745–752 (1988).
Hughes, A.J., Daniel, S.E., Ben-Shlomo, Y. & Lees, A.J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125, 861–870 (2002).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Kay, D.M. et al. Parkinson's disease and LRRK2: frequency of a common mutation in U.S. movement disorder clinics. Mov. Disord. 21, 519–523 (2006).
Devlin, B., Roeder, K. & Wasserman, L. Genomic control, a new approach to genetic-based association studies. Theor. Popul. Biol. 60, 155–166 (2001).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
McGeer, P.L., Itagaki, S., Boyes, B.E. & McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291 (1988).
Orr, C.F., Rowe, D.B., Mizuno, Y., Mori, H. & Halliday, G.M. A possible role for humoral immunity in the pathogenesis of Parkinson's disease. Brain 128, 2665–2674 (2005).
Fiszer, U., Mix, E., Fredrikson, S., Kostulas, V. & Link, H. Parkinson's disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol. Scand. 90, 160–166 (1994).
McGeer, P.L., Schwab, C., Parent, A. & Doudet, D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann. Neurol. 54, 599–604 (2003).
Langston, J.W. et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 46, 598–605 (1999).
Reynolds, A.D. et al. Regulatory T cells attenuate th17 cell-mediated nigrastriatal dopaminergic neurodegeneration in a model of Parkinson' disease. J. Immunol. 184, 2261–2271 (2010).
Barrett, J.C., Fry, B., Maller, J. & Daly, M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Acknowledgements
We would like to acknowledge the individuals with Parkinson's disease, their families and the healthy volunteers who participated in this study. We thank T.L. Edwards, J.M. Vance, E.R. Martin, J.L. Haines and M.A. Pericak-Vance for sharing their GWAS data with us; R.H. Myers, J.F. Gusella, T. Foroud and N. Pankratz for making their data public via dbGaP; and J. Degner for assistance with the eQTL data repository website at University of Chicago. We acknowledge M. Adams, M. Zilka and the staff of CIDR for excellent genotyping service, M. Palumbo, C.S. Carmack and the staff of the Computational Biology and Statistics Core of Wadsworth Center for computing support and C. Lambert and G.L. Peterson for developing the randomized plate layout. This project was supported by Award Number R01NS36960 from the National Institute of Neurological Disorders and Stroke. Additional support was provided by an Edmond J. Safra Global Genetic Consortium Grant from the Michael J. Fox Foundation for Parkinson's Disease Research, Merit Review Award from the Department of Veterans Affairs (1I01BX000531), National Institutes of Aging (P30AG08017), National Institute of Mental Health (R21MH087336), Office of Research and Development, Clinical Sciences Research and Development Service, Department of Veteran Affairs, The Intramural Research Program of the US National Institutes of Health (NIH) at the National Library of Medicine and the Close to the Cure Foundation. Genotyping services were provided by CIDR, which is fully funded through a federal contract from the NIH to Johns Hopkins University, contract number HHSN268200782096C. The work described in ref. 8, whose data were used for replication, was funded by NIH grants AG027944 and NS039764. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
H.P. established and directs the NGRC in collaboration with C.P.Z., S.A.F. and J.N. The GWAS was designed by and funded through H.P. Subjects were ascertained, diagnosed and characterized by NGRC investigators A.G., J.R., A.S., S.A.F., J.N. and C.P.Z. DNA and phenotype preparations, database operations and final subject selection for the GWAS was carried out by J.M., D.Y., D.M.K. and V.I.K. under the supervision of H.P. and C.P.Z. K.F.D. was in charge of GWAS genotyping and genotyping quality control. T.H.H. performed all statistical analyses with critical feedback from A.T., J.P., E.P. and H.P. V.I.K. and R.C. contributed to bioinformatics and graphic presentations. W.K.S. provided an independent GWAS dataset for replication. A.L. uncovered the regulatory function of rs3129882 using bioinformatics. H.P., T.H.H. and A.T. wrote the paper. All authors participated in reviewing results and assisting with manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5 and Supplementary Figures 1–6 (PDF 535 kb)
Rights and permissions
About this article
Cite this article
Hamza, T., Zabetian, C., Tenesa, A. et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet 42, 781–785 (2010). https://doi.org/10.1038/ng.642
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.642
This article is cited by
-
Modeling the neuroimmune system in Alzheimer’s and Parkinson’s diseases
Journal of Neuroinflammation (2024)
-
Unravelling the mechanisms of underweight in Parkinson’s disease by investigating into the role of gut microbiome
npj Parkinson's Disease (2024)
-
Shared genetic risk loci between Alzheimer’s disease and related dementias, Parkinson’s disease, and amyotrophic lateral sclerosis
Alzheimer's Research & Therapy (2023)
-
Phenome-wide association study on miRNA-related sequence variants: the UK Biobank
Human Genomics (2023)
-
Epigenome-wide association study of peripheral immune cell populations in Parkinson’s disease
npj Parkinson's Disease (2023)