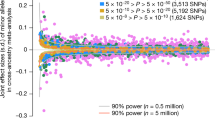
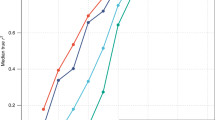
Abstract
SNPs discovered by genome-wide association studies (GWASs) account for only a small fraction of the genetic variation of complex traits in human populations. Where is the remaining heritability? We estimated the proportion of variance for human height explained by 294,831 SNPs genotyped on 3,925 unrelated individuals using a linear model analysis, and validated the estimation method with simulations based on the observed genotype data. We show that 45% of variance can be explained by considering all SNPs simultaneously. Thus, most of the heritability is not missing but has not previously been detected because the individual effects are too small to pass stringent significance tests. We provide evidence that the remaining heritability is due to incomplete linkage disequilibrium between causal variants and genotyped SNPs, exacerbated by causal variants having lower minor allele frequency than the SNPs explored to date.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
24 September 2010
In the version of this supplementary file originally posted online, Supplementary Fig. 2a and 2b were incorrect. The legend stated that in Supplementary Fig. 2a, PC1 versus PC2 was plotted when in fact PC2 versus PC3 was shown. Similarly, in Supplementary Fig. 2b, PC4 versus PC5 was plotted rather than PC3 versus PC4 as stated. This error is purely graphical and does not in any way affect the results or conclusions presented in the article. We thank Andrew Stewart for kindly pointing out this error to us. The authors regret not detecting this error earlier. The error has been corrected in this file as of 24 September 2010.
References
Hindorff, L.A. et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl. Acad. Sci. USA 106, 9362–9367 (2009).
Donnelly, P. Progress and challenges in genome-wide association studies in humans. Nature 456, 728–731 (2008).
Maher, B. Personal genomes: The case of the missing heritability. Nature 456, 18–21 (2008).
Manolio, T.A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Frazer, K.A., Murray, S.S., Schork, N.J. & Topol, E.J. Human genetic variation and its contribution to complex traits. Nat. Rev. Genet. 10, 241–251 (2009).
Pritchard, J.K. Are rare variants responsible for susceptibility to complex diseases? Am. J. Hum. Genet. 69, 124–137 (2001).
Johannes, F., Colot, V. & Jansen, R.C. Epigenome dynamics: a quantitative genetics perspective. Nat. Rev. Genet. 9, 883–890 (2008).
Johannes, F. et al. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet. 5, e1000530 (2009).
Fisher, R.A. The correlation between relatives on the supposition of Mendelian inheritance. Trans. R. Soc. Edinb. 52, 399–433 (1918).
Galton, F. Hereditary stature. Nature 33, 295–298 (1886).
Macgregor, S., Cornes, B.K., Martin, N.G. & Visscher, P.M. Bias, precision and heritability of self-reported and clinically measured height in Australian twins. Hum. Genet. 120, 571–580 (2006).
Silventoinen, K. et al. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res. 6, 399–408 (2003).
Visscher, P.M., Hill, W.G. & Wray, N.R. Heritability in the genomics era–concepts and misconceptions. Nat. Rev. Genet. 9, 255–266 (2008).
Dietz, H.C. et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352, 337–339 (1991).
Shiang, R. et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78, 335–342 (1994).
Gudbjartsson, D.F. et al. Many sequence variants affecting diversity of adult human height. Nat. Genet. 40, 609–615 (2008).
Lettre, G. et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat. Genet. 40, 584–591 (2008).
Weedon, M.N. et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat. Genet. 40, 575–583 (2008).
Visscher, P.M. Sizing up human height variation. Nat. Genet. 40, 489–490 (2008).
Hayes, B.J., Visscher, P.M. & Goddard, M.E. Increased accuracy of artificial selection by using the realized relationship matrix. Genet. Res. 91, 47–60 (2009).
Patterson, H.D. & Thompson, R. Recovery of inter-block information when block sizes are unequal. Biometrika 58, 545–554 (1971).
Zhang, X.S. & Hill, W.G. Predictions of patterns of response to artificial selection in lines derived from natural populations. Genetics 169, 411–425 (2005).
Haseman, J.K. & Elston, R.C. The investigation of linkage between a quantitative trait and a marker locus. Behav. Genet. 2, 2–19 (1972).
Visscher, P.M., Hill, W.G. & Wray, N.R. Heritability in the genomics era–concepts and misconceptions. Nat. Rev. Genet. 9, 255–266 (2008).
Slatkin, M. Epigenetic inheritance and the missing heritability problem. Genetics 182, 845–850 (2009).
Purcell, S.M. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009).
Goddard, M.E. & Hayes, B.J. Mapping genes for complex traits in domestic animals and their use in breeding programmes. Nat. Rev. Genet. 10, 381–391 (2009).
Wray, N.R., Goddard, M.E. & Visscher, P.M. Prediction of individual genetic risk to disease from genome-wide association studies. Genome Res. 17, 1520–1528 (2007).
Meuwissen, T.H., Solberg, T.R., Shepherd, R. & Woolliams, J.A. A fast algorithm for BayesB type of prediction of genome-wide estimates of genetic value. Genet. Sel. Evol. 41, 2 (2009).
McEvoy, B.P. et al. Geographical structure and differential natural selection among North European populations. Genome Res. 19, 804–814 (2009).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Dixon, W.J. Simplified estimation from censored normal samples. Ann. Math. Stat. 31, 385–391 (1960).
Acknowledgements
We are grateful to the twins and their families for their generous participation in these studies. We would like to thank staff at the Queensland Institute of Medical Research: D. Statham, A. Eldridge and M. Grace for sample collection, M. Campbell, L. Bowdler, S. Crooks and staff of the Molecular Epidemiology Laboratory for sample processing and preparation, B. Cornes for height data preparation, D. Smyth and H. Beeby for IT support and A. McRae and H. Lee for discussions. We thank N. Wray for helpful comments on the manuscript. We acknowledge funding from the Australian National Health and Medical Research Council (grants 241944, 389875, 389891, 389892, 389938, 442915, 442981, 496739, 496688 and 552485), the US National Institutes of Health (grants AA07535, AA10248, AA014041, AA13320, AA13321, AA13326 and DA12854) and the Australian Research Council (grant DP0770096).
Author information
Authors and Affiliations
Contributions
P.M.V. and M.E.G. designed the study. J.Y. performed statistical analyses. B.B., B.P.M., A.K.H., D.R.N. and S.G. performed quality control analyses and prepared data. D.R.N., P.A.M., A.C.H. and N.G.M. contributed genotype and phenotype data. J.Y., G.W.M., M.E.G. and P.M.V. contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1 and 2 and Supplementary Note (PDF 742 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Benyamin, B., McEvoy, B. et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42, 565–569 (2010). https://doi.org/10.1038/ng.608
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.608
This article is cited by
-
Temperature impacts the bovine ex vivo immune response towards Mycoplasmopsis bovis
Veterinary Research (2024)
-
Genomic dissection of the correlation between milk yield and various health traits using functional and evolutionary information about imputed sequence variants of 34,497 German Holstein cows
BMC Genomics (2024)
-
SNP-based and haplotype-based genome-wide association on drug dependence in Han Chinese
BMC Genomics (2024)
-
Investigating pedigree- and SNP-associated components of heritability in a wild population of Soay sheep
Heredity (2024)
-
Quantile generalized measures of correlation
Statistics and Computing (2024)