Abstract
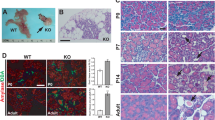
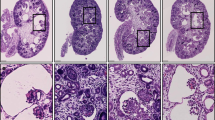
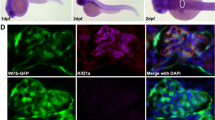
Arthrogryposis, renal dysfunction and cholestasis syndrome (ARC) is a multisystem disorder associated with abnormalities in polarized liver and kidney cells. Mutations in VPS33B account for most cases of ARC. We identified mutations in VIPAR (also called C14ORF133) in individuals with ARC without VPS33B defects. We show that VIPAR forms a functional complex with VPS33B that interacts with RAB11A. Knockdown of vipar in zebrafish resulted in biliary excretion and E-cadherin defects similar to those in individuals with ARC. Vipar- and Vps33b-deficient mouse inner medullary collecting duct (mIMDC-3) cells expressed membrane proteins abnormally and had structural and functional tight junction defects. Abnormal Ceacam5 expression was due to mis-sorting toward lysosomal degradation, but reduced E-cadherin levels were associated with transcriptional downregulation. The VPS33B-VIPAR complex thus has diverse functions in the pathways regulating apical-basolateral polarity in the liver and kidney.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
Change history
24 February 2011
In the version of this article initially published, the first and second paragraphs of the Results incorrectly stated that the C14ORF133 gene product was a previously unidentified protein and that no antibody against the C14ORF133 gene product (VIPAR) was available. In fact, an earlier study (ref. 20 in the original manuscript) reported functional analyses of the C14ORF133 gene product (also called SPE-39), described the generation of a polyclonal antibody against human SPE-39 and reported an interaction between SPE-39 and VPS33B, similar to the interaction shown in Figure 1b. These errors have been corrected in the HTML and PDF versions of the article.
References
Rodriguez-Boulan, E. & Nelson, W.J. Morphogenesis of the polarized epithelial cell phenotype. Science 245, 718–725 (1989).
Shin, K., Fogg, V.C. & Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22, 207–235 (2006).
Lubarsky, B. & Krasnow, M.A. Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28 (2003).
Anderson, J.M., Van Itallie, C.M. & Fanning, A.S. Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 16, 140–145 (2004).
Hartsock, A. & Nelson, W.J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 (2008).
Bryant, D.M. & Mostov, K.E. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887–901 (2008).
Wan, H. et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Invest. 104, 123–133 (1999).
Izaddoost, S., Nam, S.C., Bhat, M.A., Bellen, H.J. & Choi, K.W. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416, 178–183 (2002).
Ceteci, F. et al. Disruption of tumor cell adhesion promotes angiogenic switch and progression to micrometastasis in RAF-driven murine lung cancer. Cancer Cell 12, 145–159 (2007).
Wapenaar, M.C. et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut 57, 463–467 (2008).
Cullinane, A.R. et al. Molecular investigations to improve diagnostic accuracy in patients with ARC syndrome. Hum. Mutat. 30, E330–E337 (2009).
Seals, D.F., Eitzen, G., Margolis, N., Wickner, W.T. & Price, A.A. Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA 97, 9402–9407 (2000).
Starai, V.J., Hickey, C.M. & Wickner, W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol. Biol. Cell 19, 2500–2508 (2008).
Nickerson, D.P., Brett, C.L. & Merz, A.J. Vps-C complexes: gatekeepers of endolysosomal traffic. Curr. Opin. Cell Biol. 21, 543–551 (2009).
Peplowska, K., Markgraf, D.F., Ostrowicz, C.W., Bange, G. & Ungermann, C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev. Cell 12, 739–750 (2007).
Kinchen, J.M. et al. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat. Cell Biol. 10, 556–566 (2008).
Edinger, A.L., Cinalli, R.M. & Thompson, C.B. Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev. Cell 5, 571–582 (2003).
Wilkin, M. et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev. Cell 15, 762–772 (2008).
Richardson, S.C., Winistorfer, S.C., Poupon, V., Luzio, J.P. & Piper, R.C. Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol. Biol. Cell 15, 1197–1210 (2004).
Zhu, G.D. et al. SPE-39 family proteins interact with the HOPS complex and function in lysosomal delivery. Mol. Biol. Cell 20, 1223–1240 (2009).
Bogard, N., Lan, L., Xu, J. & Cohen, R.S. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development 134, 3413–3418 (2007).
Lock, J.G. & Stow, J.L. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell 16, 1744–1755 (2005).
Gissen, P. et al. Clinical and molecular genetic features of ARC syndrome. Hum. Genet. 120, 396–409 (2006).
Horslen, S.P., Quarrell, O.W. & Tanner, M.S. Liver histology in the arthrogryposis multiplex congenita, renal dysfunction, and cholestasis (ARC) syndrome: report of three new cases and review. J. Med. Genet. 31, 62–64 (1994).
Gissen, P. et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat. Genet. 36, 400–404 (2004).
Bull, L.N. et al. VPS33B mutation with ichthyosis, cholestasis, and renal dysfunction but without arthrogryposis: incomplete ARC syndrome phenotype. J. Pediatr. 148, 269–271 (2006).
Thompson, J.A., Grunert, F. & Zimmermann, W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J. Clin. Lab. Anal. 5, 344–366 (1991).
Wakabayashi, Y., Lippincott-Schwartz, J. & Arias, I.M. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and Rab11-positive endosomes. Mol. Biol. Cell 15, 3485–3496 (2004).
Nies, A.T. & Keppler, D. The apical conjugate efflux pump ABCC2 (MRP2). Pflugers. Arch. 453, 643–659 (2007).
Bandler, P.E., Westlake, C.J., Grant, C.E., Cole, S.P. & Deeley, R.G. Identification of regions required for apical membrane localization of human multidrug resistance protein 2. Mol. Pharmacol. 74, 9–19 (2008).
Takeichi, M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451–1455 (1991).
Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622–634 (2005).
Matthews, R.P. et al. Zebrafish vps33b, an ortholog of the gene responsible for human arthrogryposis-renal dysfunction-cholestasis syndrome, regulates biliary development downstream of the onecut transcription factor hnf6. Development 132, 5295–5306 (2005).
Farber, S.A. et al. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science 292, 1385–1388 (2001).
Yeaman, C. et al. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J. Cell Biol. 139, 929–940 (1997).
Matter, K. & Balda, M.S. Functional analysis of tight junctions. Methods 30, 228–234 (2003).
Aijaz, S., Sanchez-Heras, E., Balda, M.S. & Matter, K. Regulation of tight junction assembly and epithelial morphogenesis by the heat shock protein Apg-2. BMC Cell Biol. 8, 49 (2007).
Liebner, S., Kniesel, U., Kalbacher, H. & Wolburg, H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur. J. Cell Biol. 79, 707–717 (2000).
Colegio, O.R., Van Itallie, C., Rahner, C. & Anderson, J.M. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 284, C1346–C1354 (2003).
Hadj-Rabia, S. et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology 127, 1386–1390 (2004).
Dawe, H.R. et al. The Meckel-Gruber syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum. Mol. Genet. 16, 173–186 (2007).
Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002).
Brett, C.L. et al. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J. Cell Biol. 182, 1141–1151 (2008).
Keller, P., Toomre, D., Diaz, E., White, J. & Simons, K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat. Cell Biol. 3, 140–149 (2001).
Short, B., Haas, A. & Barr, F.A. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim. Biophys. Acta 1744, 383–395 (2005).
Wilcke, M. et al. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J. Cell Biol. 151, 1207–1220 (2000).
Savina, A., Fader, C.M., Damiani, M.T. & Colombo, M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6, 131–143 (2005).
Ducharme, N.A. et al. Rab11–FIP2 regulates differentiable steps in transcytosis. Am. J. Physiol. Cell Physiol. 293, C1059–C1072 (2007).
Prekeris, R. Rabs, Rips, FIPs, and endocytic membrane traffic. SciientificWorldJournal 3, 870–880 (2003).
Kikuchi, S. et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat. Genet. 31, 320–325 (2002).
Rodriguez-Boulan, E. & Müsch, A. Protein sorting in the Golgi complex: shifting paradigms. Biochim. Biophys. Acta 1744, 455–464 (2005).
Fölsch, H., Mattila, P.E. & Weisz, O.A. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic 10, 972–981 (2009).
Tuma, P.L. & Hubbard, A.L. Transcytosis: crossing cellular barriers. Physiol. Rev. 83, 871–932 (2003).
Mellman, I. & Nelson, W.J. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 9, 833–845 (2008).
Wakabayashi, Y. & Arias, I.M. Transporters on demand: intracellular reservoirs and cycling of bile canalicular ABC transporters. J. Biol. Chem. 281, 27669–27673 (2006).
Braiterman, L. et al. Apical targeting and Golgi retention signals reside within a 9-amino acid sequence in the copper-ATPase, ATP7B. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G433–G444 (2009).
Ohannesian, D.W. et al. Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin-3 in human colon carcinoma cells. Cancer Res. 55, 2191–2199 (1995).
Delacour, D. et al. Requirement for galectin-3 in apical protein sorting. Curr. Biol. 16, 408–414 (2006).
Shao, L., Allez, M., Park, M.S. & Mayer, L. Immunomodulatory roles of the carcinoembryonic antigen family of glycoproteins. Ann. NY Acad. Sci. 1072, 194–209 (2006).
Nokes, R.L., Fields, I.C., Collins, R.N. & Fölsch, H. Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells. J. Cell Biol. 182, 845–853 (2008).
Uzan-Gafsou, S. et al. Rab11A controls the biogenesis of Birbeck granules by regulating Langerin recycling and stability. Mol. Biol. Cell 18, 3169–3179 (2007).
Kass, L., Erler, J.T., Dembo, M. & Weaver, V.M. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int. J. Biochem. Cell Biol. 39, 1987–1994 (2007).
Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622–634 (2005).
Gurdon, J.B. & Bourillot, P.Y. Morphogen gradient interpretation. Nature 413, 797–803 (2001).
Goldstein, B. & Macara, I.G. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell 13, 609–622 (2007).
Langevin, J. et al. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365–376 (2005).
Shaye, D.D., Casanova, J. & Llimargas, M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat. Cell Biol. 10, 964–970 (2008).
Wang, Z. et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 (2008).
Müller, T. et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 40, 1163–1165 (2008).
Wakabayashi, Y., Dutt, P., Lippincott-Schwartz, J. & Arias, I.M. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc. Natl. Acad. Sci. USA 102, 15087–15092 (2005).
Perl, A.K., Wilgenbus, P., Dahl, U., Semb, H. & Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 (1998).
Guilford, P. et al. E-cadherin germline mutations in familial gastric cancer. Nature 392, 402–405 (1998).
Yang, J. & Weinberg, R.A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Gissen, P. et al. Comparative evolutionary analysis of VPS33 homologues: genetic and functional insights. Hum. Mol. Genet. 14, 1261–1270 (2005).
Strautnieks, S.S. et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology 134, 1203–1214 (2008).
Leung-Hagesteijn, C. et al. Integrin-linked kinase mediates bone morphogenetic protein 7-dependent renal epithelial cell morphogenesis. Mol. Cell. Biol. 25, 3648–3657 (2005).
Harlow, E. & Lane, D. Using Antibodies: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA, 1998).
Rappoport, J.Z. & Taha, B.W. Lemeer. S., Benmerah, A. & Simon, S.M. The AP-2 complex is excluded from the dynamic population of plasma membrane-associated clathrin. J. Biol. Chem. 278, 47357–47360 (2003).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 (2008).
Turner, F.E. et al. Slug regulates integrin expression and cell proliferation in human epidermal keratinocytes. J. Biol. Chem. 281, 21321–21331 (2006).
Acknowledgements
A.R.C. holds the Children Liver Disease Foundation PhD Studentship and P.G. is a GlaxoSmithKline Clinician Scientist. A.Z. and F.M. are supported by Framework 6 IP EUTRACC, LSGH CT 2006037445. H.C. is supported by grants from the European Molecular Biology Organization (ASTF 121:2007) and European Science Foundation (Exchange Grant 2008). J.Z.R. is supported by the Biotechnology and Biosciences Research Council (BB/H002308/1). Thanks to F. Lock for her help with the E-cadherin luciferase assay, G. Reynolds for help with E-cadherin immunostaining and R. Knittel for her help with freeze fracture experiments. The authors also wish to thank all the families and clinicians who contributed to this research and thank the ARC syndrome association; Children Living with Inherited Metabolic Diseases (CLIMB); Birmingham Children's Hospital Research Foundation (BCHRF); WellChild; the Wellcome Trust; and the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, US National Institutes of Health, for their generous financial support.
Author information
Authors and Affiliations
Contributions
A.R.C. designed, conducted and interpreted experiments and wrote the manuscript. A.S.-I., A.Z. and Y.W. designed, conducted and interpreted experiments. C.K.B., G.L., F.R. and H.C. performed experiments. F.G., E.U., T.B.Ö., J.D., J.V., M.D.R., H.M. contributed subjects' DNA. R.P.M., S.G.T., J.Z.R., I.M.A., H.W., A.S.K., D.A.K., F.M. and E.R.M. designed and supervised experiments. P.G. conceived and directed the project, obtained funding and wrote the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table (PDF 1815 kb)
Supplementary Movie 1
YFP-VP33B FLIP confocal live-cell microscopy. YFP-VPS33B was transfected into mIMCD-3 cells and a Fluorescence Loss In Photobleaching (FLIP) experiment was carried out in live cells. Bleaching was done using a 514 nm laser set to 100% every 5 seconds in an area of one cell, with an image captured every 2.5 seconds for a total of 5 minutes. Removal of the free cytoplasmic YFP-VPS33B pool (by photobleaching) reveals occasional cytoplasmic clusters, which are not as pronounced as when VPS33B is co-overexpressed with polarin. (WMV 1307 kb)
Supplementary Movie 2
VSVG-YFP (basolaterally targeted protein), Gal-T-GFP-, and A-VSVG-CFP infected cells were incubated overnight at 40°C. Live cell imaging was performed using a Zeiss 510 inverted confocal microscope with an ×20 objective lens. Pinhole was set fully open. Images were taken every minute. Experiment started 10min after the temperature shift to 32°C (time 0). Relative fluorescence intensities associated with the Golgi (RO1), entire cell (RO2) and plasma membrane (RO3) for one representative cell after shift to 32°C are plotted at 1min intervals. VSVG-YFP infected cells were incubated overnight at 40°C. The experiment was started 10 min after the temperature shift to 32°C (time 0). At 0 min VSVG-YFP intracellular localization of fluorescence was noted. After 140min, VSVG-YFP targeted to the plasma membrane although some intracellular fluorescence remained. Relative fluorescence intensity is calculated using the following equation: (Raw F.I. of - background F.I.) ×100/maximum F.I. (MOV 4936 kb)
Rights and permissions
About this article
Cite this article
Cullinane, A., Straatman-Iwanowska, A., Zaucker, A. et al. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet 42, 303–312 (2010). https://doi.org/10.1038/ng.538
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.538
This article is cited by
-
Ichthyosis
Nature Reviews Disease Primers (2023)
-
Novel gene mutations in three Japanese patients with ARC syndrome associated mild phenotypes: a case series
Journal of Medical Case Reports (2022)
-
Syndrome mit Schuppung und Keratosen
Der Hautarzt (2019)
-
Vps3 and Vps8 control integrin trafficking from early to recycling endosomes and regulate integrin-dependent functions
Nature Communications (2018)
-
Using Zebrafish to Model Liver Diseases-Where Do We Stand?
Current Pathobiology Reports (2017)