Abstract
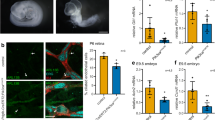
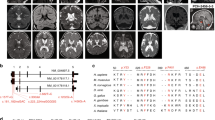
Phosphotidylinositol (PtdIns) signaling is tightly regulated both spatially and temporally by subcellularly localized PtdIns kinases and phosphatases that dynamically alter downstream signaling events1. Joubert syndrome is characterized by a specific midbrain-hindbrain malformation ('molar tooth sign'), variably associated retinal dystrophy, nephronophthisis, liver fibrosis and polydactyly2 and is included in the newly emerging group of 'ciliopathies'. In individuals with Joubert disease genetically linked to JBTS1, we identified mutations in the INPP5E gene, encoding inositol polyphosphate-5-phosphatase E, which hydrolyzes the 5-phosphate of PtdIns(3,4,5)P3 and PtdIns(4,5)P2. Mutations clustered in the phosphatase domain and impaired 5-phosphatase activity, resulting in altered cellular PtdIns ratios. INPP5E localized to cilia in major organs affected by Joubert syndrome, and mutations promoted premature destabilization of cilia in response to stimulation. These data link PtdIns signaling to the primary cilium, a cellular structure that is becoming increasingly recognized for its role in mediating cell signals and neuronal function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Vicinanza, M., D'Angelo, G., Di Campli, A. & De Matteis, M.A. Phosphoinositides as regulators of membrane trafficking in health and disease. Cell. Mol. Life Sci. 65, 2833–2841 (2008).
Valente, E.M., Brancati, F. & Dallapiccola, B. Genotypes and phenotypes of Joubert syndrome and related disorders. Eur. J. Med. Genet. 51, 1–23 (2008).
Saar, K. et al. Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am. J. Hum. Genet. 65, 1666–1671 (1999).
Valente, E.M. et al. Distinguishing the four genetic causes of Joubert syndrome-related disorders. Ann. Neurol. 57, 513–519 (2005).
Tsujishita, Y., Guo, S., Stolz, L.E., York, J.D. & Hurley, J.H. Specificity determinants in phosphoinositide dephosphorylation: crystal structure of an archetypal inositol polyphosphate 5-phosphatase. Cell 105, 379–389 (2001).
Kong, A.M. et al. Phosphatidylinositol 3-phosphate (PtdIns3P) is generated at the plasma membrane by an inositol polyphosphate 5-phosphatase: endogenous PtdIns3P can promote GLUT4 translocation to the plasma membrane. Mol. Cell. Biol. 26, 6065–6081 (2006).
Kisseleva, M.V., Cao, L. & Majerus, P.W. Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/protein kinase B phosphorylation and leads to apoptotic cell death. J. Biol. Chem. 277, 6266–6272 (2002).
Jiang, X.R. et al. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21, 111–114 (1999).
Nachury, M.V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Cantagrel, V. et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 83, 170–179 (2008).
Caspary, T., Larkins, C.E. & Anderson, K.V. The graded response to Sonic hedgehog depends on cilia architecture. Dev. Cell 12, 767–778 (2007).
Alieva, I.B., Gorgidze, L.A., Komarova, Y.A., Chernobelskaya, O.A. & Vorobjev, I.A. Experimental model for studying the primary cilia in tissue culture cells. Membr. Cell Biol. 12, 895–905 (1999).
Higginbotham, H., Bielas, S., Tanaka, T. & Gleeson, J.G. Transgenic mouse line with green-fluorescent protein-labeled Centrin 2 allows visualization of the centrosome in living cells. Transgenic Res. 13, 155–164 (2004).
Chizhikov, V.V. et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J. Neurosci. 27, 9780–9789 (2007).
Tucker, R.W., Pardee, A.B. & Fujiwara, K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell 17, 527–535 (1979).
De Donatis, A. et al. Proliferation versus migration in platelet-derived growth factor signaling: the key role of endocytosis. J. Biol. Chem. 283, 19948–19956 (2008).
Schneider, L. et al. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861–1866 (2005).
Pugacheva, E.N., Jablonski, S.A., Hartman, T.R., Henske, E.P. & Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351–1363 (2007).
Santagata, S. et al. G-protein signaling through tubby proteins. Science 292, 2041–2050 (2001).
Rohatgi, R. & Scott, M.P. Arrestin' movement in cilia. Science 320, 1726–1727 (2008).
Valente, E.M. et al. AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann. Neurol. 59, 527–534 (2006).
Murray, S.S. et al. A highly informative SNP linkage panel for human genetic studies. Nat. Methods 1, 113–117 (2004).
Hoffmann, K. & Lindner, T.H. easyLINKAGE-Plus—automated linkage analyses using large-scale SNP data. Bioinformatics 21, 3565–3567 (2005).
Gleeson, J.G. et al. Genetic and neuroradiological heterogeneity of double cortex syndrome. Ann. Neurol. 47, 265–269 (2000).
Valente, E.M. et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 38, 623–625 (2006).
Inglis, P.N., Boroevich, K.A. & Leroux, M.R. Piecing together a ciliome. Trends Genet. 22, 491–500 (2006).
Gherman, A., Davis, E.E. & Katsanis, N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat. Genet. 38, 961–962 (2006).
Caldwell, K.K., Lips, D.L., Bansal, V.S. & Majerus, P.W. Isolation and characterization of two 3-phosphatases that hydrolyze both phosphatidylinositol 3-phosphate and inositol 1,3-bisphosphate. J. Biol. Chem. 266, 18378–18386 (1991).
Vandeput, F., Backers, K., Villeret, V., Pesesse, X. & Erneux, C. The influence of anionic lipids on SHIP2 phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase activity. Cell. Signal. 18, 2193–2199 (2006).
Zhang, X., Hartz, P.A., Philip, E., Racusen, L.C. & Majerus, P.W. Cell lines from kidney proximal tubules of a patient with Lowe syndrome lack OCRL inositol polyphosphate 5-phosphatase and accumulate phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 273, 1574–1582 (1998).
Kisseleva, M.V., Wilson, M.P. & Majerus, P.W. The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. J. Biol. Chem. 275, 20110–20116 (2000).
Rodriguez, L.G., Wu, X. & Guan, J.L. Wound-healing assay. Methods Mol. Biol. 294, 23–29 (2005).
Kim, J., Krishnaswami, S.R. & Gleeson, J.G. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 17, 3796–3805 (2008).
Acknowledgements
We thank the Marshfield Clinic Research Foundation, Center for Inherited Disease Research and University of California Los Angeles Microarray Core (supported by the US National Heart, Lung, and Blood Institute and National Institutes of Health) for genotyping support. J. Meerloo at the University of California San Diego (UCSD) Neuroscience Microscopy Imaging Core (P30NS047101) provided imaging support. Ryan Anderson of the UCSD Material Sciences provided electron microscopy support. We thank members of the the Dixon lab (UCSD) for suggestions and help with protein modeling, and members of the Mitchell lab (Monash University) for reagents. This work was supported by the UCSD Neuroplasticity of Aging Training Grant (to S.L.B.), the Italian Ministry of Health (RC2008 to B.D., Ricerca Finalizzata 2006 to E.M.V.), the Telethon Foundation Italy (GGP08145 to E.B. and E.M.V.), National Institutes of Health grant HL 16634 (to P.W.M. and M.V.K.), American Heart Association grant 0730350N (to M.V.K.), the National Institute of Neurological Disorder and Stroke, the Burroughs Welcome Fund, the March of Dimes and the Howard Hughes Medical Institute (to J.G.G.).
Author information
Authors and Affiliations
Contributions
S.L.B, J.L.S, F.B., L.C.S., L.T., S.G., M.J., S.S. and M.V.K. performed experiments. L.A.-G., L.S., M.S.Z, A.A.-A., O.R., H.K., D.S., L.C.S., E. Bertini, E. Boltshauser and E.F. identified and recruited patients. R.A.B. shared unpublished data and reagents. S.J.F., B.D. and P.W.M. provided advice and helped with data interpretation. S.L.B and J.L.S assembled the figures. S.L.B, E.M.V. and J.G.G. wrote and edited the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–3 (PDF 1857 kb)
Rights and permissions
About this article
Cite this article
Bielas, S., Silhavy, J., Brancati, F. et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41, 1032–1036 (2009). https://doi.org/10.1038/ng.423
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.423
This article is cited by
-
Deletion of Aurora kinase A prevents the development of polycystic kidney disease in mice
Nature Communications (2024)
-
Inpp5e Regulated the Cilium-Related Genes Contributing to the Neural Tube Defects Under 5-Fluorouracil Exposure
Molecular Neurobiology (2024)
-
Primary cilia as dynamic and diverse signalling hubs in development and disease
Nature Reviews Genetics (2023)
-
PIP2 determines length and stability of primary cilia by balancing membrane turnovers
Communications Biology (2022)
-
Phosphoinositides as membrane organizers
Nature Reviews Molecular Cell Biology (2022)