Abstract
Outcomes of hepatitis C virus (HCV) infection and treatment depend on viral and host genetic factors. Here we use human genome-wide genotyping arrays and new whole-genome HCV viral sequencing technologies to perform a systematic genome-to-genome study of 542 individuals who were chronically infected with HCV, predominantly genotype 3. We show that both alleles of genes encoding human leukocyte antigen molecules and genes encoding components of the interferon lambda innate immune system drive viral polymorphism. Additionally, we show that IFNL4 genotypes determine HCV viral load through a mechanism dependent on a specific amino acid residue in the HCV NS5A protein. These findings highlight the interplay between the innate immune system and the viral genome in HCV control.
Similar content being viewed by others
Main
HCV infection presents a major health burden, with more than 185 million people being infected worldwide1, which can lead to liver failure and hepatocellular cancer in infected individuals. Genetic variations in both the host and the virus are associated with important clinical outcomes. Genetic polymorphisms in the host, most notably in the interferon (IFN) lambda 3 (IFNL3) and IFNL4 loci, are associated with spontaneous clearance of the virus, response to treatment, viral load and progression of liver disease2,3,4,5,6. Viral genotypes and distinct viral genetic motifs have been associated with the response to interferon-based therapies7,8, whereas resistance-associated substitutions (RASs) have been identified for most of the new oral direct-acting antiviral (DAA) drugs9,10,11,12. HCV can be divided into seven major genotypes, and most of the genetic data acquired to date has focused on HCV genotype 1, with a lack of data for other genotypes. HCV genotype 3 is of particular interest, as this genotype is known to infect 53 million people globally13 and is associated with a higher failure rate to DAA therapies14,15.
Previous work, including candidate gene studies of the association between the human leukocyte antigen (HLA) type I proteins and the HCV genome16,17, has shown that within-host virus diversity evolves in response to the host adaptive immune system. HLA molecules are expressed on most cell types, and they present viral peptides (epitopes) to cytotoxic T lymphocytes (CTLs), which kill infected cells. CTL-mediated killing of virus-infected cells drives the selection of viral polymorphisms ('escape' mutations) that abrogate T cell recognition18. Understanding how host HLA molecules affect viral selection has important implications for the development of HCV-specific T cell vaccines that aim to prevent infection19,20. A comprehensive host genome to viral genome analysis at scale will assess the relative contribution of host HLA molecules in driving changes in the HCV genome, and it might also identify other host genes that have a key role in shaping the HCV genome.
We generated data from a cohort of 601 HCV-infected patients (from the BOSON21 clinical trial) to systematically look for associations between host and virus genomes, exploiting the fact that while the host genome remains fixed the virus mutates, allowing it to evolve during infection. For this, we developed a targeted viral enrichment methodology22,23 to obtain whole HCV genomes, and we used high-throughput genotyping arrays in combination with statistical imputation to obtain data for nucleotide polymorphisms across the human genome and the alleles of genes encoding HLA molecules24,25 (hereafter referred to as HLA genes). We provide evidence that polymorphisms relevant to the innate (IFNL4) and adaptive immune systems (HLA genes) are associated with HCV sequence polymorphisms. We show that an interaction between host IFNL4 genotypes and an amino acid residue in the HCV NS5A protein determines HCV viral load. By assessing viral evolution in individuals with different IFNL4 genotypes, we highlight systematic differences in the innate immune response and discuss how these might relate to previous associations with spontaneous clearance and clinical treatment. We demonstrate the potential for a joint analysis of host and viral genomic data to provide information on underlying molecular interactions and their importance in treating and preventing HCV, as well as other viral infections, in the era of genomic analysis.
Results
Sample description and genetic structure
DNA samples from 567 patients (of 601 patients) were genotyped using the Affymetrix UK Biobank array. This array genotypes over 800,000 single-nucleotide polymorphisms (SNPs) across the human genome, including a set of markers specifically chosen to capture common HLA alleles. Pretreatment plasma samples from 583 patients in the same study were analyzed to obtain consensus HCV whole-genome sequences using a high-throughput HCV-targeted sequence-capture approach coupled with Illumina sequencing22.
Both full-length HCV genome sequences and human genome-wide SNP data were obtained on a total of 542 patients of mainly self-reported white and Asian ancestry who were infected with HCV genotypes 2 or 3 (Supplementary Table 1). After quality control analysis and filtering of the human genotype data, approximately 330,000 common SNPs with a minor allele frequency >5% were available for analysis, along with inferred alleles at both class I and II HLA genes. The full-length HCV genome is approximately 9.5 kb, which corresponds to >3,000 encoded amino acids. In our data set, we defined 1,226 sites of the HCV proteome to be variable (where at least ten isolates had an amino acid that differed from the consensus amino acid, giving adequate statistical power for analysis).
We characterized human genetic diversity in the cohort via principal component analysis (PCA). The first two principal components (PCs) corresponded to the sample's (self-reported) 83% white and 14% Asian ethnicity (Supplementary Fig. 1), which differed significantly (P < 0.05) in some of the inferred HLA allele frequencies (Supplementary Table 2), consistent with previous observations26. The third PC separates individuals of self-reported black ethnicity from the rest of the cohort. We summarized virus diversity by constructing a maximum-likelihood tree of the consensus sequences from each patient (Supplementary Fig. 2). Major clades in the tree separated HCV genotypes 2 (8% of the total number of samples) and 3 (which in turn comprised clades representing samples with subtype 3a (90% of the samples) and non-subtype 3a (2% of the samples)). We observed that patients of specific ancestries, as measured either by genetic ancestry (PCs) or self-reported ethnicity, clustered together on the tree of viral diversity (Supplementary Fig. 2). A PCA of virus nucleotide sequence data reflected the structure of the tree, specifically at the level of virus subtypes (Supplementary Fig. 3).
Systematic host genome to virus genome analysis
We used the genotyped autosomal SNPs in the host genome to undertake genome-wide association studies, for which the traits of interest were the presence or absence of each amino acid at the variable sites of the virus proteome, which resulted in nearly one billion association tests. We performed logistic regression assuming an additive model and adjusting for sex and population structure by including the first three PCs of the host and the first ten PCs of the virus as covariates. Failure to control for either of the covariates led to a substantial inflation in the association test statistics (Supplementary Fig. 4), as would be expected given the observed correlation in population structure of the virus and the host (Supplementary Fig. 2). We assumed that there was a human genome-wide significance threshold27 of 5 × 10−8 and that amino acid variants in the viral genome were approximately uncorrelated once the population structure was accounted for, and we found that a Bonferroni correction28 resulted in a significance threshold of approximately 2 × 10−11.
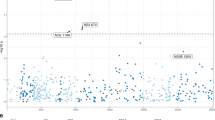
Across the human genome, the most significant associations were observed between multiple SNPs in the major histocompatibility complex (MHC) locus (on chromosome 6) and a virus amino acid variant in non-structural protein 3 (NS3) (Fig. 1). Three other associations were observed between multiple SNPs in the host MHC locus and the virus amino acids in the NS3 and NS4B proteins (P < 4 × 10−9; Fig. 1). Outside of the MHC locus, the strongest association between host and virus was detected between the SNP rs12979860 in the host IFNL4 gene (on chromosome 19) and the amino acids at position 2,570 in the HCV NS5B protein (P = 1.98 × 10−9; Fig. 1). The observed variability in the density of nominally significant associations (Fig. 1) can largely be explained by variability in the host and virus sequences, for example, in the hypervariable region (HVR) of the HCV E2 protein.
The lower arc shows the human autosomes from chromosomes 1 to 22, and the upper arc shows the HCV proteome from the core protein (C) to NS5B. The red line represents the most significant association (P < 2 × 10−11). The four blue lines represent suggestive associations (P < 4 × 10−9). The thin gray lines represent associations with P < 10−5. The outer mini-panels represent, on the upper arc, the viral diversity as measured by Shannon entropy and, on the lower arc, the density of human SNPs in bins of 1 Mb, with higher values further away from the center for both the upper and lower arcs.
We observed 182 associations between human SNPs that mapped to two loci and five HCV amino acid sites (P < 4 × 10−9) (Supplementary Table 3). Because these associations represent places where host genetic diversity has had an effect on virus sequence diversity, we refer to them as 'footprints'. We interpreted the signals of association in the MHC region to indicate the effect of the adaptive immune system on genetic diversity in the virus genome. Although the effect of MHC was anticipated, the strong signal of association of the interferon lambda region with viral diversity indicated that there were additional effects of the innate immune response.
Host HLA alleles to virus genome
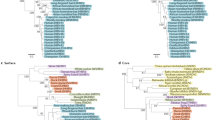
SNPs in the MHC-encoding region that show strong association with viral amino acids are likely to be correlated with alleles at the HLA genes due to extensive linkage disequilibrium across the region29,30. The HLA repertoire of a patient defines which viral peptides will be presented to T cells as part of the adaptive immune response, and this mechanism can lead to the selection of viral escape mutations16,31,32,33,34. Upon transmission to another host with a different HLA repertoire, reversion to the wild type may occur ('reversion mutations'). To test for footprinting of the host HLA alleles along the viral genome (Fig. 2a and Table 1), we inferred the changes on the terminal branches of the virus phylogenetic tree for each amino acid site and assessed their association with the hosts' HLA alleles. We repeated the analysis on patients who were infected with HCV genotype 3a and who self-reported as white (Table 1 and Supplementary Fig. 5).
(a–c) Association between HCV amino acids (on x axis) and HLA alleles (by Fisher's exact test) (a), IFNL4 genotypes (by Fisher's exact test) (b) and pre-treatment viral load (log10(PTVL); by linear regression) (c). Sites in experimentally validated epitopes in HCV genotype 3 are indicated by a plus sign. Viral sites at positions 1,444, 2,414 and 2,570 are colored red. Dashed lines represent a 5% FDR. (d) Schematic of the HCV polyprotein.
In the whole cohort, at a false discovery rate (FDR) of 5%, 24 combinations of HLA alleles and HCV sites were significant (Table 1), and this increased to 153 associations at a FDR of 20% (Supplementary Table 4). Of 21 viral amino acid positions that showed signals of association with one or more HLA alleles, 12 were located in previously reported HCV genotype 3 epitopes20 (Table 1), which represents a strong enrichment (odds ratio (OR) = 5.2; P = 2.8 × 10−4). We also observed that the NS3 protein was strongly enriched for association signals with HLA alleles (OR = 5.5; P = 2.2 × 10−4). The strongest HLA footprints were found with common alleles at HLA-A and HLA-B, although signals were also found in HLA-C and the class II gene HLA-DQA1 (see Supplementary Note and Supplementary Figs. 6,7,8 for a detailed description of the most significant association between HLA-A and Tyr1,444Phe). At amino acid position 1,646 in the HCV polyprotein (in the NS3 protein), the footprint is seen with multiple HLA alleles (HLA-B*08:01, HLA-C*07:01 and HLA-DQA1*05:01), although this is potentially a result of linkage disequilibrium between these HLA alleles (r2 between HLA-B*08:01 and HLA-C*07:01 = 0.49, and r2 between HLA-B*08:01 and HLA-DGA1*05:01 = 0.22; Supplementary Fig. 9). At a 20% FDR, using the 27 HLA and viral amino acid associations that have sufficient observations to estimate the ORs, we observed a negative correlation between the ORs of escape and reversion amino acids (r = –0.65; P < 0.01; Supplementary Fig. 10). These observations are consistent with HLA alleles driving patterns of both escape and reversion at viral amino acids. Our analysis provides a map of their influence across the HCV genome.
IFNL4 host variants to virus genome
Variants in the interferon lambda region have been associated with multiple HCV outcomes, including spontaneous clearance, treatment response, viral load and liver disease progression2,3,35. In our genome-wide analysis, outside of the MHC region, variants around the interferon lambda region showed the strongest association with HCV amino acids (the top associated SNP is rs12979860, P = 1.98 × 10−9). For SNP rs12979860, the CC genotype is associated with higher rates of spontaneous clearance and interferon-based treatment response, putatively owing to the fact that the C allele tags a dinucleotide insertion polymorphism (rs368234815-TT) that prevents IFNL4 expression36. In our cohort, only two individuals had discordant genotypes for rs12979860 and rs368234815 (which was imputed), resulting in a strong linkage disequilibrium (r2 = 0.99) between these two SNPs. Therefore, individuals with non-CC genotypes express IFNL4 and have increased expression of hepatic interferon-stimulated genes (ISGs)37.
We compared viral amino acid changes between hosts with CC and non-CC genotypes at the IFNL4 SNP rs12979860, using the same Fisher's exact test as described above. The most significant association was with changes to and away from valine at position 2,570 in the virus NS5B protein (P = 1.94 × 10−11; Fig. 2b). We replicated this association in an independent study of 360 patients of European ancestry who were chronically infected with HCV genotypes 2 or 3 (one-sided P = 0.005)38,39. In addition, a candidate gene association study in a genotype 1b single-source infection cohort has shown an association between IFNL4 genotypes (rs12979860) and an amino acid in the same region of NS5B (position 2,609)32, reinforcing the potential role of the locus in interactions with the host innate immune system.
Overall, using a 5% FDR, we observed 11 significant associations between IFNL4 genotypes and amino acid positions located in the HCV core, E2, NS5A and NS5B proteins (Supplementary Table 5). Using a permutation approach, we found that the core protein was nominally (P < 0.05) enriched, and the NS5A protein was nominally depleted, in the number of associations with the IFNL4 genotypes. Genotype 3a viral sequences in individuals with the CC genotype did have more mutations away from the population consensus than individuals with the non-CC genotype (Supplementary Note and Supplementary Table 6). One of the sites (position 109 in the core protein) was also associated with HLA-B*41:02 (P = 4.3 × 10−6; Table 1). However, we did not find any consistent evidence for interaction between IFNL4 genotypes, the HLA alleles and their associated viral amino acid sites (Supplementary Fig. 11) nor did we find strong differences in the mean number of escape mutations in patients with the CC or non-CC genotypes when comparing across all combinations of HLA alleles and viral sites associated at 20% FDR.
To further investigate the selective pressures on the virus in patients with IFNL4 CC and non-CC genotypes, we estimated the rates of synonymous (dS) and nonsynonymous (dN) substitutions in patients infected with HCV genotype 3a (Fig. 3). Although there was no difference in the dS values in patients with the CC or non-CC genotype (P = 0.68; Fig. 3a), the dN value was significantly higher in patients with the CC genotype (P = 1.6 × 10−8; Fig. 3b). The lower dN/dS ratio in patients with the non-CC genotype (P = 1.3 × 10−10) is potentially indicative of the idea that the virus is under a stronger purifying selection than in patients with the CC genotype (Fig. 3c). This hypothesis is supported by the observation that, for the same rate of synonymous substitutions dS (a surrogate for the time and amount of divergence), the HCV genome is under a larger purifying selection in patients with IFNL4 non-CC genotypes than in patients with the CC genotype (Fig. 3d).
(a) Rate of synonymous substitutions (dS, P = 0.68; by linear regression). (b) Rate of nonsynonymous substitutions (dN, P = 1.6 × 10−8; by linear regression). (c) The dN/dS ratio (P = 1.28 × 10−10; by linear regression). In a–c, the mean and 95% confidence intervals are shown as black dots and bars, respectively. (d) The joint distribution of dS and dN/dS values in individuals with either the IFNL4 non-CC genotypes (red dots) or the IFNL4 CC genotype (blue dots).
Estimating dN/dS ratio per viral gene showed that this ratio was significantly higher (P < 0.05) in CC patients compared to non-CC patients in E1, E2, NS3 and NS5B (Supplementary Fig. 12). A sliding window analysis across the HCV genome showed that in the full cohort E1 and E2 genes had a much higher dN/dS ratio compared to the rest of the genome. These envelope genes include hyper-variable regions (HVRs), and are thought to be the primary targets of the antibody-based immune response (Supplementary Fig. 13).
Host and virus genetic determinants of viral load
We performed a genome-wide association study (GWAS) in patients who were infected with HCV genotype 3a, using an additive linear regression model adjusted for sex and the three first host PCs for log10-transformed pre-treatment viral load (log10(PTVL)) (Fig. 4a). We replicated the known association between IFNL4 variants on chromosome 19 and viral load3 (rs12979860, P = 5.9 × 10−10), with the non-CC genotypes conferring an approximately 0.45-fold decrease in viral load (mean for non-CC = 3.4760 × 106 IU/ml, and for CC = 6.3447 × 106 IU/ml). We also performed a GWAS to detect associations between amino acids of HCV genotype 3a and viral load (Fig. 2c). The only amino acid that was significantly associated with log10(PTVL) at a 5% FDR was residue 2,414 (in the NS5A protein), which showed a change from a serine to an asparagine (P = 9.21 × 10−7; Fig. 4b). This site is one of the 11 sites that were significantly associated (5% FDR) with IFNL4 genotypes (Fig. 2b,c).
(a) Association between human SNPs and log10(PTVL). (b–d) Distribution (blue dots), estimated mean and 95% confidence interval (shown as black dots and bars, respectively) of log10(PTVL) stratified by the amino acids present at position 2,414 in HCV (P = 9.21 × 10−7; by linear regression) (b), by IFNL4 genotypes (CC, CT and TT) in patients whose virus carries a serine at position 2,414 (P = 9.37 × 10−9; by linear regression) (c) or in patients whose virus does not carry a serine at position 2,414 (P = 0.9; by linear regression) (d).
In patients infected with HCV that had a serine at position 2,414, the association between IFNL4 genotypes and log10(PTVL) was significant (P = 9.37 × 10−9; Fig. 4c). However, we observed no association (P = 0.9) between IFNL4 genotypes and log10(PTVL) in patients who were infected with a virus that had a different amino acid at that position (Fig. 4d). In other words, the host's IFNL4 genotypes determined viral load only if the individuals were infected by a virus with a serine at position 2,414 in the NS5A protein (Fig. 4c,d). The interaction was statistically significant when analyzing either the whole cohort (P = 0.017) or just patients who had been infected with HCV genotype 3a and who self-reported as being white (P = 0.017). Taken together, the combinations of patients with non-CC genotypes and HCV with serine at site 2,414 are inferred to result in a 0.57-fold decrease in viral load as compared to that for all other combinations (mean viral load for non-CC and serine at site 2,414 = 2.81 × 106 IU/ml, and mean viral load for all other combinations = 6.47 × 106 IU/ml). Introduction of the change from a serine to an asparagine at position 2,414 into a modified S52 replicon of HCV genotype 3a (ref. 40) resulted in an approximately tenfold increase in replication of the replicon in Huh7.5 cell culture (Supplementary Note and Supplementary Fig. 14).
We also observed that non-consensus amino acids, which are increased in frequency in individuals with a CC genotype, tended also to associate with increased viral load (r = 0.42; P = 0.005; Supplementary Fig. 15). The same positive relationship was observed when estimating the effect on viral load in individuals with a CC genotype only (r = 0.35; P = 0.02) or with non-CC genotypes only (r = 0.4; P = 0.007). A nominally significant trend was observed when the analysis was repeated for all of the variant positions in the viral genome (r = 0.099; P = 0.04) (Supplementary Fig. 16).
Discussion
Here we report the first systematic analysis of associations between variation in human and HCV genomes in a large patient cohort. Advances in DNA and RNA sequencing technology and new bioinformatics tools have allowed full-length viral consensus sequences to be obtained in large numbers of patients for a reasonable cost (∼£100 per sample), as well as host genetic data at millions of directly assayed and imputed polymorphisms (∼£75 per sample). We apply a fast and simple approach to test for association between host and pathogen variants, using a logistic regression analysis corrected for both human and viral population structures by using the PCs of the genome-wide data as covariates (60 h for ∼2,500 association studies of 330,000 SNPs). We also applied a contingency table analysis based on the inferred amino acid changes in the virus after infection. We anticipate that with the reduction in the cost of sequencing and genotyping, and the increasing interest in studying large patient cohorts, analyses of this kind will become a powerful approach to understanding infectious diseases. Confirmation of the specific associations reported here, and the extent to which they are specific to viral genotype, will require replication analysis in independent cohorts.
We found strong evidence for the adaptive immune system exerting selective pressures on the HCV genome, presumably by preferentially selecting for viral mutations that avoid antigenic presentation by the host's HLA proteins. Some of the observed associations are located in experimentally validated viral epitopes20; however, others have not been described experimentally and most likely represent sites of previously unknown T cell escape mutations. Assuming that our analysis removes biases associated with population structure and incorrect ancestral inference, 5% of the viral amino acids (153/3,021) are associated with HLA alleles (at 20% FDR). These data highlight the importance of the adaptive immune system in driving viral evolution and serve as a map of the targets of T cell–based immunity along the HCV genome, which can aid vaccine design and development20.
In addition to the HLAs expressed by an individual, we now show that host IFNL4 activity may significantly shape the HCV viral genome. Previous studies have shown that the 'favorable' CC IFNL4 genotype increases the chances of spontaneous resolution and interferon-based treatment success2,3,4,5,6. The IFNL4 TT/TT genotype (rs368234815), which is strongly linked to the favorable CC genotype (rs12979860), abolishes the expression of IFNL4 (ref. 36), whereas in individuals with the IFNL4 ΔG/TT or ΔG/ΔG genotypes (which are linked to 'unfavorable' non-CC genotypes), IFNL4 is expressed, leading to the downstream upregulation of hepatic ISG expression via the JAK–STAT signaling pathway37. The expression of ISGs has been shown to render the host less susceptible to stimulation by exogenously added IFN-α and IFN-γ, and it is associated with more infected cells in the liver41. To date, it has been presumed that this fully explains why patients with specific IFNL4 genotypes have differential outcomes during primary infection or with drug therapy41,42,43.
Our analysis adds to this hypothesis with the observation that IFNL4 variants also have an effect on the HCV genome at multiple amino acid sites. Indeed, these were the strongest footprinting signals in our systematic analysis outside of the HLA region. The most significant association was at residue 2,570 in the viral NS5B protein, an association that we replicated in an independent cohort, whose members were infected with HCV genotypes 2 and 3. The additional signals in the NS5B protein that were associated with IFNL4 genotypes (or HLA alleles) at a 5% FDR showed no significant association (P > 0.05) in our replication cohort, potentially due to a lack of power resulting from the smaller sample size of the replication cohort and/or the fact that the phylogenetically corrected Fisher's exact tests could not be performed in an equivalent manner (only NS5B sequences were available in the replication cohort). An association between IFNL4 genotypes and amino acid variability in the same region of the NS5B protein has previously been reported in a candidate gene study32 in a cohort with a single-source infection. IFNL4 has also been associated with a viral mutation associated with DAA drug resistance in HCV genotype 1 infection44, although this association has not been replicated in our HCV genotype 3–infected cohort45. However, the broader effect of the interferon lambda host genes that are associated with, and potentially select for, specific viral variants has not been previously recognized.
HCV viral load is an important and clinically relevant parameter, as patients with higher HCV viral loads have lower response rates to IFN- and DAA-drug-based therapy46 (independent of IFNL4 status). Paradoxically, the favorable IFNL4 variants have also been associated with both an increase in disease progression47 and high viral load3,48. We report an association between a virus amino acid site (serine versus non-serine at position 2,414 in NS5A) and HCV viral load. This site is one of the 11 sites that are putatively associated with IFNL4 genotypes in our data set. Furthermore, our data show that a decrease in viral load was observed only in those patients with non-CC genotypes whose virus encoded a serine at amino acid residue 2,414. Because the Ser2,414 variant is found in 85% of patients with the non-CC genotypes (as compared to 67% of patients with the CC genotype), this interplay between host and viral genes helps explain the previous observation that patients with the non-CC genotypes have a lower HCV viral load (Fig. 5). Our in vitro data from a genotype 3 replicon assay showed that a change from a serine to asparagine at site 2,414 was associated with an increase in RNA replication and perhaps hyper-phosphorylation of the HCV NS5A protein49, which is a negative regulator of virus replication.
(a) Infected individuals who have the IFNL4 CC genotype (37% of the HCV genotype 3a–infected patients) show high rates of spontaneous and treatment-induced clearance of HCV. IFNL4 is not expressed, which in turn induces a weaker, and possibly differential, expression of ISGs. The host environment is associated with weaker purifying selection and allows viral mutations associated with a better replicative fitness to accumulate, leading to higher viral load. In this group of patients, 67% were infected by a virus with a serine at position 2,414 and 33% with a different amino acid at that position. (b) Infected individuals with IFNL4 non-CC genotypes (63% of the HCV genotype 3a–infected patients) had lower rates of spontaneous and treatment-induced clearance of HCV. IFNL4 is expressed and induces expression of ISGs that collectively establish an antiviral state that is hostile to viral replication. This hostile environment induces a high selective pressure, and fewer viral mutations can accumulate. Although the serine at position 2,414 (as compared to non-serine residues) is associated with lower viral load, it is highly prevalent in this group of patients (85% are infected by a virus with a serine at position 2,414 and 15% with a different amino acid at that position).
Our interpretation of the data (Fig. 5) is that the expression of IFNL4 by the ΔG/TT or ΔG/ΔG genotypes (tagged in our cohort by the 'unfavorable' non-CC genotypes) leads to the activation of additional components of the immune response, likely driven by ISGs, which interact directly with specific amino acids encoded by the viral genome (most notably amino acid 2,414 in NS5A, which has a significant impact on viral load; Figs. 2b and 4 and Supplementary Fig. 15). Our data suggest that this also leads to an overall increase in the strength of purifying selection (decrease in dN/dS; Fig. 3), and together this leads to lower viral load. However, viruses that establish chronic infections in patients with non-CC genotypes have evolved to survive in a more hostile environment (for example, by mutating the Ser2,414 of NS5A), which makes them less likely to respond to interferon-based therapy. In contrast, our analysis suggests that the favorable CC genotype and the inactivation of the IFNL4 gene (by the IFNL4 TT genotype) disables components of the immune response (therefore removing the effect of amino acid residue 2,414 in NS5A on viral load), which leads to a reduced level of purifying selection (Fig. 3). It is possible that this then permits a range of mutations that confer higher replicative fitness and, therefore, higher viral load (Supplementary Fig. 15), but these viruses are more susceptible to interferon-based treatments. At the population level, we would expect a balance in the relative contribution of these mechanisms as viruses move between individuals with CC and non-CC genotypes. Our results make the prediction that the outcome of a new infection will be dependent on both the HLA alleles and the IFNL4 genotype of the patient who is the source of the new infection. Further analysis is required to fully understand the effect of favorable CC and unfavorable non-CC genotypes on the different components of the immune system and to establish their clinical relevance before, during and after infection.
In conclusion, we provide a comprehensive genome-to-genome analysis in chronic HCV infection. By using this genome-wide, hypothesis-free approach, we show that the host's HLA alleles leave multiple footprints in the HCV genome and that the host's innate immune environment also influences the amino acid polymorphisms in the virus, both at specific loci and genome wide. We observe a common viral amino acid residue that is associated with HCV viral load in only patients with the unfavorable non-CC IFNL4 genotypes. These observations suggest that the innate and adaptive immune systems jointly have an effect on HCV genome evolution and together probably determine the establishment of infection and its control over time. The new insights into the biological mechanisms that drive HCV evolution in vivo and the identification of specific interactions between viral and host polymorphisms are relevant for future approaches to treatment stratification and vaccine development.
Methods
Patients and samples.
Plasma and DNA samples came from patients enrolled in the BOSON study. The BOSON study is a phase 3 randomized open-label trial to determine the efficacy and safety of treatment with sofosbuvir, with and without pegylated IFN-α, in treatment-experienced patients with cirrhosis and HCV genotype 2 infection and treatment-naive or treatment-experienced patients with HCV genotype 3 infection21. All patients provided written informed consent before undertaking any study-related procedures. The study protocol was approved by each institution's review board or ethics committee before study initiation. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines and the Declaration of Helsinki. The study reported here is not a clinical trial, but is based on the analysis of patients from a clinical trial (registration number: NCT01962441). Sample sizes were determined by the available data. All samples for which both viral sequencing and host genetics were available were included in the final analysis unless otherwise specified.
Host genotyping and imputation.
Informed consent for host genetic analysis was obtained from 567 patients. Genotyping was performed using Affymetrix UK Biobank arrays. We imputed the MHC class I loci HLA-A, HLA-B and HLA-C and the MHC class II loci HLA-DQA1, HLA-DQB1, HLA-DPB1, HLA-DRB1, HLA-DRB3, HLA-DRB4 and HLA-DRB5 using HLA*IMP:02 (accessed 22 March 2015)24. HLA amino acids were also imputed by SNP2HLA50 using the T1DGC as the reference panel, which contains data from 5,225 unrelated individuals (10,450 haplotypes). Logistic regression using posterior genotype probabilities (allele dosages) for each HLA allele from SNP2HLA were carried out using PLINK2 (https://www.cog-genomics.org/plink2)51.
Virus sequencing.
Sample collection and preparation. RNA was isolated from 500 μl plasma using the NucliSENS magnetic extraction system (bioMerieux) and collected in 30 μl of kit elution buffer for storage at –80 °C in aliquots.
Sequencing library construction, enrichment and sequencing. Libraries were prepared for Illumina sequencing using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England BioLabs) with 5 μl sample (maximum 10 ng total RNA) and previously published modifications of the manufacturer's guidelines (v2.0)22, including fragmentation for 5 min at 94 °C, omission of actinomycin D at first-strand reverse transcription, library amplification for 18 PCR cycles using custom indexed primers52 and post-PCR clean-up with 0.85× volume Ampure XP (Beckman Coulter).
Libraries were quantified using Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen) and analyzed using Agilent TapeStation with D1K High Sensitivity Kit (Agilent) for equimolar pooling; they were then re-normalized by qPCR using the KAPA SYBR FAST qPCR Kit (Kapa Biosystems) for sequencing. A 500-ng aliquot of the pooled library was enriched using the xGen Lockdown protocol from Integrated DNA Technologies (IDT) (Rapid Protocol for DNA Probe Hybridization and Target Capture Using an Illumina TruSeq or Ion Torrent Library (v1.0)) with equimolar-pooled 120-nt DNA oligonucleotide probes (IDT) followed by a 12-cycle, modified, on-bead, post-enrichment PCR re-amplification step. The cleaned post-enrichment library was normalized with the aid of qPCR and sequenced with 151-base paired-end reads on a single run of the Illumina MiSeq using v2 chemistry.
Sequence data analysis. De-multiplexed sequence-read pairs were trimmed of low-quality bases using QUASR (v7.0120)53 and of adaptor sequences using CutAdapt (version 1.7.1)54, and they were subsequently discarded if either the read had less than 50 bases of remaining sequence or if both reads matched the human reference sequence using Bowtie (version 2.2.4)55. The remaining read pool was screened against a BLASTn database containing 165 HCV genomes56, which covered its diversity both to choose an appropriate reference and to select those reads that formed a population for de novo assembly with Vicuna (v1.3)57. The assembly was finished with V-FAT v1.0 (http://www.broadinstitute.org/scientific-community/science/projects/viral-genomics/v-fat). The population consensus sequence at each site was defined as the most common variant at that site among all of the patients.
Phylogenetic and ancestral sequence reconstruction. Whole-genome viral consensus sequences for each patient were aligned using MAFFT58 with default settings. This alignment was used to create a maximum-likelihood tree using RAxML59, assuming a general time-reversible model of nucleotide substitution under the gamma model of rate heterogeneity. The resulting tree was rooted at midpoint. Maximum-likelihood ancestral sequence reconstruction was performed using RAxML59 with the maximum-likelihood tree and HCV polyprotein sequences as input.
Association analysis.
To test for association between human SNPs and HCV amino acids at the genome-to-genome level, we performed logistic regression using PLINK2 (https://www.cog-genomics.org/plink2)51, adjusted for the human population structure (three first PCs as assessed using EIGENSOFT v3.0)60 and the virus population structure (ten first PCs). For the viral data, PCA was performed on the nucleotide data as follows. Tri- and quad-allelic sites were converted to binary variables, and the amino acid frequencies were standardized to have mean zero and unit variance. MATLAB (release 2015a, The MathWorks) was used to perform the PCA using the singular value decomposition function.
To test for association between imputed HLA alleles and HCV amino acids, we used Fisher's exact test, correcting for the virus population structure as described in Bhatacharaya et al.61. We used the inferred ancestral amino acid to estimate changes for each site on the terminal branches of the virus phylogenetic tree. Inferring the changes along the terminal branches of the tree aims to control for the confounding between host and virus population structures61 by looking at viral mutations after infection. We constructed a 2 × 4 contingency table, where rows denoted presence or absence of a host HLA allele, and the columns denoted changes to and away from a specific amino acid in viruses, with and without the amino acid inferred to be ancestral. To test for association between IFNL4 SNP rs12979860 genotypes and HCV amino acids, we used the same Fisher's exact test with a dominant model for IFNL4 rs12979860 by encoding genotypes as CC or non-CC.
Permutation was used to estimate the FDR for association tests that used Fisher's exact test. The rows of matrix M (where rows corresponded to study participants and columns to HLA alleles or the IFNL4 genotypes) were randomly permuted 500 times, and in each case the P-values of the associations were calculated. For each threshold t, the expected number of false significant associations was estimated by the mean number of false positives across permuted null data sets. The FDR for a threshold t was then estimated as the mean number of false positives divided by the observed number of significant associations in the actual data at threshold t.
To test for the enrichment of association signals in epitope regions, viral proteins or with a specific HLA allele, we used Fisher's exact test. Each site was either within a target region (epitope regions or a specific viral protein) or not, and the most associated test with it was significant or not with an FDR of 5%. The resulting contingency table was tested using Fisher's exact test to assess enrichment or depletion of signals of association.
To assess the relationship between rates of escape and reversion in HLA presentation, we estimated the odds ratio for each 2 × 2 subtable used in the Fisher's exact test. This was done only for viral sites associated with HLA alleles at 20% FDR and for which there were a sufficient number of observations in both tables to estimate the odds ratio (OR) for which the confidence interval did not go to infinity. Pearson's correlation coefficient was used to assess the relationship between the log10(OR) of escape and reversion.
To test for enrichment of viral amino acid associations with host IFNL4 genotypes in viral proteins, the null distribution of the number of associations in each protein was estimated using 10,000 permutations of IFNL4 labels and performing the same tests. The estimated null distribution of the number of associations for each viral protein was compared to the observed number of associations in the data to test for enrichment or depletion of the number of associations. To test whether the hypervariable region 1 (HVR1), HVR2, HVR3, the interferon-sensitivity-determining region (ISDR) and the RNA-dependent protein kinase–binding domain (PKR-BD) region showed differences in the number of changes away from the population consensus in hosts with CC or non-CC genotypes, we used a Poisson regression analysis. For each individual and each locus, we determined the number of differences relative to the population consensus. We then estimated the effect of IFNL4 genotypes on the mean number of differences relative to the population consensus using Poisson regression. The same procedure was used to test whether the total number of differences across the whole polyprotein relative to the population consensus was influenced by IFNL4 genotypes.
To estimate the rate of synonymous and non-synonymous mutations, we used the dndsml function from MATLAB (release 2015a, The MathWorks) that uses Goldman and Yang's method. It estimates (using maximum likelihood) an explicit model for codon substitution that takes into account transition and transversion rate bias, as well as base and codon frequency bias. It then uses the model to correct synonymous and non-synonymous counts to account for multiple substitutions at the same site. To estimate dN and dS, each sequence was compared to the population consensus sequence, which indicates the most common nucleotide observed in our data set at each position along the genome.
To determine whether IFNL4 genotypes have an effect on HLA allele presentation of epitopes, we used logistic regression with an interaction term. The outcome variable for each individual was whether there was any change in the specific amino acid on the terminal branch of the virus tree associated with that individual. We tested for interaction between presence or absence of the associated HLA allele and IFNL4 genotypes for all of the combinations of HLA alleles and viral sites associated at a 20% FDR. In addition, we tested for an overall effect of IFNL4 genotypes on the HLA alleles' presentation of epitopes. We used our 2 × 4 contingency tables and the ORs estimated from the 2 × 2 subtables to infer the antigenic amino acids. If the OR indicated that in individuals with HLA allele present the 'X ancestral amino acid any other amino acid' element was enriched relative to individuals without the HLA allele, then we assumed that the X amino acid was the antigenic amino acid and that escape occurred away from X to any other amino acid. For these cases, we count how many of 'X ancestral amino acid any other amino acid' incidences occurred in individuals with CC or non-CC genotypes across all combinations of HLA alleles and viral sites associated at 20% FDR. If the IFNL4 genotypes have no effect on HLA presentation (null hypothesis), then the mean number of escape mutations in hosts with the CC or non-CC genotypes should be proportional to the frequency of hosts with the CC or non-CC genotypes (null distribution is binomial with parameters n equal to the total number of observed escape mutations and p equal to the proportion of hosts with the CC genotype).
We used linear regression in PLINK2 (https://www.cog-genomics.org/plink2)51 to test for association between human SNPs and log10-transformed pretreatment viral load (PTVL), including sex and the first three PCs of the host genome as covariates. We used linear regression to test for association between HCV amino acids (with a minimal count of ten at each site) and viral load. We used linear regression in R version 3.2.4 (2016-03-10)62 to analyze the interactions between IFNL4 genotypes and amino acids at viral site 2,414 and to quantify their effect on viral load.
For all of the viral sites associated with IFNL4 genotypes at 20% FDR, we assessed whether there was a relationship between the effect size of non-consensus amino acids on viral load and the effect size of IFNL4 genotypes and changes to non-consensus amino acids. We estimated the OR of enrichment of changes away from the consensus amino acid on the terminal branches of the virus tree in individuals with CC and non-CC genotypes (2 × 2 contingency table; using only data in which the consensus was inferred to be ancestral). We also estimated the effect size of non-consensus amino acids on the log10 value of viral load using a linear regression analysis with IFNL4 genotype as a covariate. Additionally, we estimated the effect size of the non-consensus amino acids on the log10(viral load) in hosts with CC or non-CC genotypes. We used Pearson's correlation coefficient to measure the strength of the relationship between the effect size of non-consensus amino acids on viral load and the log of the OR of enrichment of non-consensus amino acid changes in hosts with a CC genotype relative to that in hosts with non-CC genotypes.
Code availability.
The R and MATLAB code used to generate the results and figures from the primary analyses described above are available from the authors on request.
Replication study.
To replicate the IFNL4 SNP rs12979860 results, we ran the association analysis on an independent population of HCV-infected individuals that was recruited to the FISSION, FUSION and POSITRON phase 3 clinical studies38,39. The material transfer agreements under which the data were shared limited analysis to the NS5B protein. Paired human genome-wide genotyping and HCV Sanger sequencing data for the NS5B amplicon were obtained from DNA and plasma samples collected from 360 self-reported white patients who were chronically infected with HCV genotype 2 (n = 153) or HCV genotype 3 (n = 208). We searched for association between the IFNL4 SNP rs12979860 and viral amino acid position 2,570 using logistic regression with the outcome indicated by the presence or absence of a valine at that residue. To help prevent spurious associations due to host and viral stratification, we included human PCs and viral genotype as covariates.
Replicon assay.
Cell culture. Huh7.5-Sec14L2 human cells, obtained from J. Witteveldt40, were grown in Dulbecco's modified Eagle's medium (DMEM, Life Technologies) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 mM HEPES and 0.1 M non-essential amino acids as described63. The genotype of Huh7.5 cell line for the IFNL4 SNP (rs12979860) is heterozygous CT64.
HCV mutant replicons. The enhanced version of the subgenomic replicon of genotype 3a strain S52, which lacks the neomycin resistance gene, has been previously described40. Site-specific mutations were introduced using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies), following the manufacturer's instructions, and confirmed by direct sequencing. Plasmids with mutated HCV sequences were linearized by XbaI digestion (New England BioLabs; NEB), treated with mung bean nuclease (NEB) and purified. Linearized DNA was then used as a template for in vitro RNA transcription (IVT) (Megascript T7, Life Technologies) according to the manufacturer's protocol. Finally, the IVT RNA was DNAse-treated, purified and stored at –80 °C.
Electroporation and luciferase detection. For electroporation, cells were counted and then washed twice in ice-cold PBS. Typically, for each mutant, 4 × 106 cells were mixed with 1 μg of replicon RNA in a 4-mm cuvette and electroporated using the Gene Pulser Xcell (Bio-Rad) at 250 V, 950 μF and the exponential-decay setting. Cells were immediately recovered in pre-warmed complete DMEM, seeded in a 24-well plate and incubated at 37 °C. After 5, 24, 48 or 72 h, medium was removed and cells were lysed with Glo Lysis Buffer (Promega). Cell lysates were then transferred in a white 96-well plate (Corning), and the amount of luciferase expression was quantified in a luminometer (GloMax 96 Microplate Luminometer, Promega) using the Bright-Glo assay system (Promega).
Data availability.
Human genotype data underlying this manuscript are deposited in the European Genome–phenome Archive under accession code EGAS00001002324. HCV sequence data underlying this manuscript are deposited in GenBank under accession codes KY620313–KY620880. Information on access to the study data is available at http://www.stop-hcv.ox.ac.uk/data-access.
References
Mohd Hanafiah, K., Groeger, J., Flaxman, A.D. & Wiersma, S.T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57, 1333–1342 (2013).
Thomas, D.L. et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801 (2009).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment–induced viral clearance. Nature 461, 399–401 (2009).
Rauch, A. et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology 138, 1338–1345 (2010).
Suppiah, V. et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nat. Genet. 41, 1100–1104 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41, 1105–1109 (2009).
Enomoto, N. et al. Mutations in the nonstructural protein 5a gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N. Engl. J. Med. 334, 77–82 (1996).
Pascu, M. et al. Sustained virological response in hepatitis C virus type 1b–infected patients is predicted by the number of mutations within the NS5A-ISDR: a meta-analysis focused on geographical differences. Gut 53, 1345–1351 (2004).
Halfon, P. & Locarnini, S. Hepatitis C virus resistance to protease inhibitors. J. Hepatol. 55, 192–206 (2011).
Halfon, P. & Sarrazin, C. Future treatment of chronic hepatitis C with direct-acting antivirals: is resistance important? Liver Int. 32, 79–87 (2012).
Ahmed, A. & Felmlee, D.J. Mechanisms of hepatitis C viral resistance to direct-acting antivirals. Viruses 7, 6716–6729 (2015).
Sarrazin, C. et al. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B or NS3, and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology 151, 501–512 (2016).
Messina, J.P. et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 61, 77–87 (2015).
Pol, S., Vallet-Pichard, A. & Corouge, M. Treatment of hepatitis C virus genotype 3 infection. Liver Int. 34 (Suppl. 1), 18–23 (2014).
Ampuero, J., Romero-Gómez, M. & Reddy, K.R. HCV genotype 3—the new treatment challenge. Aliment. Pharmacol. Ther. 39, 686–698 (2014).
Fitzmaurice, K. et al. Molecular footprints reveal the impact of the protective HLA-A*03 allele in hepatitis C virus infection. Gut 60, 1563–1571 (2011).
Neumann-Haefelin, C. et al. Human leukocyte antigen B27 selects for rare escape mutations that significantly impair hepatitis C virus replication and require compensatory mutations. Hepatology 54, 1157–1166 (2011).
Heim, M.H. & Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 61 (Suppl. 1), S14–S25 (2014).
Swadling, L. et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 6, 261ra153 (2014).
von Delft, A. et al. The broad assessment of HCV genotypes 1 and 3 antigenic targets reveals limited cross-reactivity with implications for vaccine design. Gut 65, 112–123 (2016).
Foster, G.R. et al. Efficacy of sofosbuvir plus ribavirin, with or without peginterferon-α, in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 149, 1462–1470 (2015).
Bonsall, D. et al. ve-SEQ: robust, unbiased enrichment for streamlined detection and whole-genome sequencing of HCV and other highly diverse pathogens. F1000 Res. 4, 1062 (2015).
Thomson, E. et al. Comparison of next-generation sequencing technologies for the comprehensive assessment of full-length hepatitis C viral genomes. J. Clin. Microbiol. 54, 2470–2484 (2016).
Dilthey, A. et al. Multi-population classical HLA type imputation. PLoS Comput. Biol. 9, e1002877 (2013).
Zheng, X. et al. HIBAG—HLA genotype imputation with attribute bagging. Pharmacogenomics J. 14, 192–200 (2014).
Gonzalez-Galarza, F.F. et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease, and HLA adverse drug reaction associations. Nucleic Acids Res. 43, D784–D788 (2015).
Pe'er, I., Yelensky, R., Altshuler, D. & Daly, M.J. Estimation of the multiple-testing burden for genome-wide association studies of nearly all common variants. Genet. Epidemiol. 32, 381–385 (2008).
Johnson, R.C. et al. Accounting for multiple comparisons in a genome-wide association study (GWAS). BMC Genomics 11, 724 (2010).
de Bakker, P.I.W. et al. A high-resolution HLA and SNP haplotype map for disease-association studies in the extended human MHC. Nat. Genet. 38, 1166–1172 (2006).
Malkki, M., Single, R., Carrington, M., Thomson, G. & Petersdorf, E. MHC microsatellite diversity and linkage disequilibrium among common HLA-A, HLA-B, DRB1 haplotypes: implications for unrelated donor hematopoietic transplantation and disease-association studies. Tissue Antigens 66, 114–124 (2005).
Ruhl, M. et al. CD8+ T cell response promotes evolution of hepatitis C virus nonstructural proteins. Gastroenterology 140, 2064–2073 (2011).
Merani, S. et al. Effect of immune pressure on hepatitis C virus evolution: insights from a single-source outbreak. Hepatology 53, 396–405 (2011).
Rauch, A. et al. Divergent adaptation of hepatitis C virus genotypes 1 and 3 to human leukocyte antigen–restricted immune pressure. Hepatology 50, 1017–1029 (2009).
Gaudieri, S. et al. Evidence of viral adaptation to HLA class I–restricted immune pressure in chronic hepatitis C virus infection. J. Virol. 80, 11094–11104 (2006).
Patin, E. et al. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology 143, 1244–1252 (2012).
Prokunina-Olsson, L. et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 45, 164–171 (2013).
Terczyńska-Dyla, E. et al. Reduced IFN-λ4 activity is associated with improved HCV clearance and reduced expression of interferon-stimulated genes. Nat. Commun. 5, 5699 (2014).
Jacobson, I.M. et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 368, 1867–1877 (2013).
Lawitz, E. et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 368, 1878–1887 (2013).
Witteveldt, J., Martin-Gans, M. & Simmonds, P. Enhancement of the replication of hepatitis C virus replicons of genotypes 1 to 4 by manipulation of CpG and UpA dinucleotide frequencies and use of cell lines expressing SECL14L2 for antiviral resistance testing. Antimicrob. Agents Chemother. 60, 2981–2992 (2016).
Sheahan, T. et al. Interferon-λ alleles predict innate antiviral immune responses and hepatitis C virus permissiveness. Cell Host Microbe 15, 190–202 (2014).
Bibert, S. et al. IL28B expression depends on a novel TT/–G polymorphism, which improves HCV clearance prediction. J. Exp. Med. 210, 1109–1116 (2013).
Ferraris, P. et al. Cellular mechanism for impaired hepatitis C virus clearance by interferon associated with IFNL3 gene polymorphisms relates to intrahepatic interferon-λ expression. Am. J. Pathol. 186, 938–951 (2016).
Peiffer, K.-H. et al. Interferon-λ4 genotypes and resistance-associated variants in patients infected with hepatitis C virus genotypes 1 and 3. Hepatology 63, 63–73 (2016).
Pedergnana, V. et al. Interferon-λ4 variant rs12979860 is not associated with RAV NS5A Y93H in hepatitis C virus genotype 3a. Hepatology 64, 1377–1378 (2016).
McHutchison, J.G. et al. Peginterferon-α2b or α2a with ribavirin for treatment of hepatitis C infection. N. Engl. J. Med. 361, 580–593 (2009).
Bochud, P.-Y. et al. IL28B alleles associated with poor hepatitis C virus (HCV) clearance protect against inflammation and fibrosis in patients infected with non-1 HCV genotypes. Hepatology 55, 384–394 (2012).
Thompson, A.J. et al. Interleukin 28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 139, 120–129 (2010).
Tellinghuisen, T.L., Foss, K.L. & Treadaway, J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 4, e1000032 (2008).
Jia, X. et al. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS One 8, e64683 (2013).
Chang, C.C. et al. Second-generation PLINK: rising to the challenge of larger and richer data sets. Gigascience 4, 7 (2015).
Lamble, S. et al. Improved workflows for high-throughput library preparation using the transposome-based Nextera system. BMC Biotechnol. 13, 104 (2013).
Gaidatzis, D., Lerch, A., Hahne, F., Stadler, M.B. & Quas, R. Quantification and annotation of short reads in R. Bioinformatics 31, 1130–1132 (2015).
Martin, M. Cutadapt removes adapter sequences from high-throughput-sequencing reads. EMBnet.journal 17, 10 (2011).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Smith, D.B. et al. Expanded classification of hepatitis C virus into seven genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology 59, 318–327 (2014).
Yang, X. et al. De novo assembly of highly diverse viral populations. BMC Genomics 13, 475 (2012).
Katoh, K. & Standley, D.M. MAFFT multiple-sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Bhattacharya, T. et al. Founder effects in the assessment of HIV polymorphisms and HLA allele associations. Science 315, 1583–1586 (2007).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2016).
Magri, A. et al. Rethinking the old antiviral drug moroxydine: discovery of novel analogues as anti–hepatitis C virus (HCV) agents. Bioorg. Med. Chem. Lett. 25, 5372–5376 (2015).
Rojas, Á. et al. Hepatitis C virus infection alters lipid metabolism depending on IL28B polymorphism and viral genotype, and modulates gene expression in vivo and in vitro. J. Viral Hepat. 21, 19–24 (2014).
Acknowledgements
We thank Gilead Sciences for providing samples and data from the BOSON clinical study for use in these analyses. We also thank HCV Research UK (funded by the Medical Research Foundation) for their assistance in handling and coordinating the release of samples for these analyses.
This work was funded by a grant from the Medical Research Council (MRC) (MR/K01532X/1; to the STOP-HCV Consortium). The work was supported by Core funding to the Wellcome Trust Centre for Human Genetics provided by the Wellcome Trust (090532/Z/09/Z; C.C.A.S. and R.B.). E.B. is funded by the MRC as an MRC Senior Clinical Fellow and received additional support from the NIHR Oxford BRC and the Oxford Martin School. M.A.A. is funded by the Oxford Martin School. G.C. is funded by the BRC of the Imperial College NHS Trust. P.K. is funded by the Oxford Martin School, the NIHR Oxford BRC, the Wellcome Trust (109965MA) and the US National Institutes of Health (U19AI082630). J.M. has funding support from the MRC (MC.UU.12014/1) and the Medical Research Foundation (C0365). C.C.A.S. is funded by Wellcome Trust grant 097364/Z/11/Z. G.M. is funded by Wellcome Trust grant 100956/Z/13/Z.
Author information
Authors and Affiliations
Consortia
Contributions
M.A.A. and V.P. contributed equally to this work, and E.B. and C.C.A.S. jointly supervised this research. M.A.A., V.P., E.B. and C.C.A.S. conceived and designed the experiments; M.A.A., V.P., C.L.C.I., A.M., D.B., A.V.D., D.S., N.C., I.B., A.T. and P.P. performed the experiments; M.A.A., V.P., A.M., N.C., I.B., G.N. and C.C.A.S. performed the statistical analysis; M.A.A., V.P., A.M., D.B., P.K., E.B. and C.C.A.S. analyzed the data; C.L.C.I., G.N., A.V.D., D.B., D.S., G.M., A.T., P.P., J.F. and J.M. contributed reagents, materials and analysis tools; and M.A.A., V.P., J.F., G.C., G.R.F., E.H., J.M., P.S., R.B., P.K., E.B. and C.C.A.S. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
G.R.F. has received grants from, consulted for, has been a speaker at or has been on the advisory board of AbbVie, Alcura, Bristol-Myers Squibb, Gilead, Janssen, GlaxoSmithKline, Merck, Roche, Springbank, Idenix, Tekmira and Novartis. G.M. is a partner in Peptide Groove LLP, which commercializes the HLA*IMP software used for HLA imputation.
Integrated supplementary information
Supplementary Figure 1 Ancestry stratifies patterns of human genetic variations.
PCA plots (1st, 2nd and 3rd components) estimated from human genotypes. Individuals are coloured by self-reported population of origin. Because of the over-representation of individuals from the respective populations, the 1st and 2nd components differentiate South Asian (Pakistan, India) and Western European patients, while the 3rd component differentiates a small number of patients with African ancestry from the other populations.
Supplementary Figure 2 Virus whole-genome phylogeny labeled by self-reported ethnicity for all patients.
Maximum-likelihood phylogenetic tree was estimated from virus whole-genome sequences using RAxML software with a general time reversible substitution model incorporating a gamma model of rate heterogeneity and a constrained guide tree. The resulting tree was mid-point rooted. The tips of the tree are coloured by self reported host ethnicities. White, Asian and others are coloured respectively as red, blue and grey on the tips of the tree.
Supplementary Figure 3 PCA of the virus nucleotides is associated with HCV subtypes.
PCA plots (1st to 10th components) estimated from virus whole-genome sequences. HCV genotypes were inferred by comparing sequences with HCV whole genome reference sequences. Blue dots represent HCV genotype 3 (circles represent subtype 3a, triangles subtype 3b, square represents subtype 3g, crosses represent subtype 3i); green dots represent genotype 2 (circles represent subtype 2a, triangles subtype 2b, squares represent subtype 2c, cross represents subtype 2m); the red circle represents the 1a/2b recombinant isolate.
Supplementary Figure 4 QQplot for genome-to-genome analysis.
Top panel) No adjustment for population structure. Bottom panel) Adjusted for human (first 3 PCs) and virus (first 10 PCs) population structures.
Supplementary Figure 5 Scatter plot of -log10(P) of association tests performed in the White ancestry patient infected with genotype 3a virus against the whole cohort.
Top) Host HLA alleles and viral amino acids association study. Middle) IFNL4 genotypes (rs12979860, CC vs. non-CC) and viral amino acids association study. Bottom) Viral load and viral amino acids association study. Our phylogenetically corrected Fisher’s exact test accounts for the confounding effect of population structure of the host and the virus, by estimating the within patient evolution of the virus. Performing the tests in a subset of the data where the host and viral ancestry are homogenous, results in highly correlated P-values for small P-values.
Supplementary Figure 6 Association between viral position 1444 and HLA-A alleles.
(a) Table of changes to and from the ancestral amino acids in individuals with and without the HLA-A*01:01 allele. (b) Estimated effect size (log odds ratio) of common HLA-A alleles and the top associated SNP (rs3129073) on the possibility of mutation from F to Y at viral position 1444. The black dots represent the effect size estimate and the bar represent the 95% confidence intervals.
Supplementary Figure 7 Sequence logo representations of the virus nucleotide and the amino acid profiles around position 1444.
The dots represent the association between the human variants (SNP rs3095267 (blue), HLA-A*01:01 (green) and amino acid N77del in HLA-A protein (red)) and the viral variants (nucleotides (triangle) and amino acids (circle)). The green region indicates the experimentally validated epitope for HLA-A*01 allele. SNPs and HLA amino acids in the MHC region were imputed using SNP2HLA program. While rs3129073 is the most associated genotyped SNP with Y1444F; rs3095267 is the most associated imputed SNP.
Supplementary Figure 9 Linkage disequilibrium between the HLA alleles.
Only alleles that present at least one pairwise r2>0.5 are shown here.
Supplementary Figure 10 Bi-directionality of escape and reversion for viral amino acid sites associated with HLA alleles at 20% FDR.
Here we plotted the log10 of odds ratio of the first part of the 2x4 contingency table against the log10 of odds ratio of the second part of the table only for sites that had sufficient observations to estimate the odds ratio of escape and reversion. An escape mutation away from the wild type amino acid in the people with the matched HLA alleles is usually associated with fitness cost for the virus. Therefore it is expected that upon transmission to people without that HLA allele, for the escape mutation to reverse back to wild type. This is the assumption of bi-directionality. There is a negative correlation (R=-0.65, P<0.01) in the log10 of odds ratio of escape and reversion.
Supplementary Figure 11 Interaction between IFNL4 genotypes and HLA allele escape mutations.
For the sites associated with HLA alleles at a 20% FDR, we tested for interaction between IFNL4 genotypes and HLA allele using a logistic regression. Each dot is the -log10(P) for the interaction term between IFNL4 genotypes and the HLA allele associated with the amino acids at the site.
Supplementary Figure 12 Association between dN/dS and IFNL4 genotypes at the viral gene level.
For each viral gene the rate of synonymous and non-synonymous mutations were estimated for all isolates. The difference in the mean dN/dS in CC and non-CC patients is nominally significant in E1, E2 NS3 and NS5B genes.
Supplementary Figure 13 Sliding window analysis of dN and dS along viral genome.
Using a window size of 31 nucleotides, moving one codon at a time, the pairwise rate of synonymous and non-synonymous mutations were estimated. The green regions from left to right indicate HVR1, HVR2 ISDR and HVR3 regions. The red lines indicate the 11 sites associated with IFNL4 genotypes. Top panel) Mean rates of synonymous and non-synonymous mutations for all genotype 3a isolates. Middle panel) Mean dN/dS ratio for IFNL4 CC and non-CC individuals. Bottom panel) Difference in mean of the dN/dS for IFNL4 CC and non-CC individuals. The difference in mean is higher in CC individuals above 0 (log10(P) is plotted) and is higher in non-CC individuals below 0 (-log10(P) is plotted).
Supplementary Figure 14 RNA replication of S52 ΔN genotype 3a HCV replicon including amino acid changes at a subset of sites associated with IFNL4 and nominally associated with viral load.
Top panel, the difference in replication relative to the un-modified S52 ΔN replicon of two sites with modified amino acids associated with IFNL4 genotypes. Middle panel, the difference in replication relative to the un-modified S52 ΔN replicon of eight sites with modified amino acids nominally associated with viral load. Bottom panel, Time course of S52 ΔN replicon modified at site 2414 which is associated both with IFNL4 genotypes and viral load. A change from a serine (S) to an asparagine (N) resulted in a 10-fold increase in viral replication. GND is the negative control sample with an inactivated RNA polymerase and S52 is the un-modified replicon. Y-axis is the luciferase assay in normalized raw light units (RLU).
Supplementary Figure 15 Viral amino acids affect on viral load vs. IFNL4 odds ratio of escape from consensus for viral sites associated with IFNL4 genotypes at a 20% FDR.
We only plotted sites that had at least 10 non-consensus amino acids in each of IFNL4 CC and non-CC genotypes. On x-axis we have plotted the log10 of odds ratio for the contingency table where rows are the counts of CC and non-CC patients and the columns are the counts for the change and no change on the terminal branches of the tree from the most common amino acid (log10(OR) > 0 is interpreted as proportion of changes from “most common amino acid” to “any other amino acid” is higher in CC patients than non-CC patients). On y-axis the effect size of non-consensus amino acids (relative to consensus amino acid) on viral load is plotted (estimated using linear regression where in the top panel the IFNL4 genotypes are added as a covariate). In the bottom two panels, the effect size of the non-consensus amino acid on viral load was estimated only in IFNL4 CC and non-CC patients. The red dot represents the viral position 2414, associated with IFNL4 genotypes and viral load.
Supplementary Figure 16 Viral amino acids affect on viral load vs. IFNL4 odds ratio of escape from consensus for viral sites associated with IFNL4 genotypes for all sites.
We only plotted sites that had at least 10 non-consensus amino acids in each of IFNL4 CC and non-CC genotypes. On x-axis we have plotted the log10 of odds ratio for the contingency table where rows are the counts of CC and non-CC patients and the columns are the counts for the change and no change on the terminal branches of the tree from the most common amino acid (log10(OR) > 0 is interpreted as proportion of changes from “most common amino acid” to “any other amino acid” is higher in CC patients than non-CC patients). On y-axis the effect size of non-consensus amino acids (relative to consensus amino acid) on viral load is plotted (estimated using linear regression where in the top panel the IFNL4 genotypes are added as a covariate). In the bottom two panels, the effect size of the non-consensus amino acid on viral load was estimated only in IFNL4 CC and non-CC patients. The red dot represents the viral position 2414, associated with IFNL4 genotypes and viral load.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16, Supplementary Tables 1–6 and Supplementary Note (PDF 4015 kb)
Rights and permissions
About this article
Cite this article
Ansari, M., Pedergnana, V., L C Ip, C. et al. Genome-to-genome analysis highlights the effect of the human innate and adaptive immune systems on the hepatitis C virus. Nat Genet 49, 666–673 (2017). https://doi.org/10.1038/ng.3835
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3835
This article is cited by
-
Host genetic regulation of human gut microbial structural variation
Nature (2024)
-
Genome-wide host-pathogen analyses reveal genetic interaction points in tuberculosis disease
Nature Communications (2023)
-
Direct-acting antiviral resistance of Hepatitis C virus is promoted by epistasis
Nature Communications (2023)
-
Molecular evolution in different subtypes of multifocal hepatocellular carcinoma
Hepatology International (2023)
-
The role of IFNL4 in liver inflammation and progression of fibrosis
Genes & Immunity (2022)