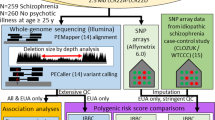
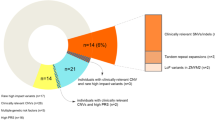
Centre for Applied Genomics and Program in Genetics and Genome Biology, The Hospital for Sick Children, Toronto, Ontario, Canada
Christian R Marshall, Daniele Merico, Bhooma Thiruvahindrapuram, Zhouzhi Wang & Stephen W Scherer
Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, Massachusetts, USA
Daniel P Howrigan, Stephan Ripke, Brendan Bulik-Sullivan, Kai-How Farh, Menachem Fromer, Jacqueline I Goldstein, Hailiang Huang, Phil Lee, Mark J Daly & Benjamin M Neale
Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA
Daniel P Howrigan, Stephan Ripke, Richard A Belliveau Jr, Sarah E Bergen, Elizabeth Bevilacqua, Brendan Bulik-Sullivan, Kimberley D Chambert, Menachem Fromer, Giulio Genovese, Phil Lee, Colm O'Dushlaine, Edward M Scolnick, Jordan W Smoller, Mark J Daly, Steven A McCarroll, Jennifer L Moran, Aarno Palotie, Tracey L Petryshen & Benjamin M Neale
Beyster Center for Psychiatric Genomics, University of California, San Diego, La Jolla, California, USA.,
Wenting Wu, Douglas S Greer, Danny Antaki, Aniket Shetty, Madhusudan Gujral, William M Brandler, Dheeraj Malhotra, Karin V Fuentes Fajarado, Michelle S Maile & Jonathan Sebat
Department of Psychiatry, University of California, San Diego, La Jolla, California, USA.,
Wenting Wu, Douglas S Greer, Danny Antaki, Aniket Shetty, Madhusudan Gujral, William M Brandler, Dheeraj Malhotra, Karin V Fuentes Fajarado, Michelle S Maile & Jonathan Sebat
MRC Centre for Neuropsychiatric Genetics and Genomics, Institute of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University, Cardiff, UK
Peter A Holmans, Noa Carrera, Nick Craddock, Valentina Escott-Price, Lyudmila Georgieva, Marian L Hamshere, David Kavanagh, Sophie E Legge, Andrew J Pocklington, Alexander L Richards, Douglas M Ruderfer, Nigel M Williams, George Kirov, Michael J Owen, James T R Walters & Michael C O'Donovan
National Centre for Mental Health, Cardiff University, Cardiff, UK
Peter A Holmans, Nick Craddock, Alexander L Richards, Michael J Owen & Michael C O'Donovan
Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Dalila Pinto, Guiqing Cai, Kenneth L Davis, Elodie Drapeau, Joseph I Friedman, Vahram Haroutunian, Elena Parkhomenko, Abraham Reichenberg, Jeremy M Silverman & Joseph D Buxbaum
Department of Genetics and Genomic Sciences, Seaver Autism Center, Mindich Child Health and Development Institute, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Dalila Pinto
Neuroscience Discovery and Translational Area, Pharma Research and Early Development, F. Hoffmann-La Roche, Ltd, Basel, Switzerland
Dheeraj Malhotra & Enrico Domenici
NORMENT, KG Jebsen Centre for Psychosis Research, Institute of Clinical Medicine, University of Oslo, Oslo, Norway
Ingrid Agartz, Srdjan Djurovic, Morten Mattingsdal, Ingrid Melle, Ole A Andreassen & Erik G Jönsson
Department of Psychiatry, Diakonhjemmet Hospital, Oslo, Norway
Ingrid Agartz
Department of Clinical Neuroscience, Psychiatry Section, Karolinska Institutet, Stockholm, Sweden
Ingrid Agartz, Erik Söderman & Erik G Jönsson
State Mental Hospital, Haar, Germany
Margot Albus
Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California, USA
Madeline Alexander, Claudine Laurent & Douglas F Levinson
Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, Georgia, USA
Farooq Amin
Department of Psychiatry and Behavioral Sciences, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia, USA
Farooq Amin
School of Biomedical Sciences and Pharmacy, University of Newcastle, Callaghan, New South Wales, Australia
Joshua Atkins, Murray J Cairns, Rodney J Scott, Paul A Tooney & Jing Qin Wu
Hunter Medical Research Institute, New Lambton, New South Wales, Australia
Joshua Atkins
Department of Psychiatry, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, Virginia, USA
Silviu A Bacanu, Tim B Bigdeli, Mark A Reimers, Bradley T Webb, Aaron R Wolen, Brandon K Wormley, Kenneth S Kendler & Brien P Riley
Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
Sarah E Bergen, Anna K Kähler, Patrik K E Magnusson, Christina M Hultman & Patrick F Sullivan
Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Mental Health Services Copenhagen, Copenhagen, Denmark
Marcelo Bertalan, Thomas Hansen, Line Olsen, Henrik B Rasmussen & Thomas Werge
Lundbeck Foundation Initiative for Integrative Psychiatric Research, iPSYCH, Denmark
Marcelo Bertalan, Thomas Hansen, Manuel Mattheisen, Line Olsen, Henrik B Rasmussen & Thomas Werge
Department of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA
Donald W Black
Department of Psychiatry, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Richard Bruggeman
School of Nursing, Louisiana State University Health Sciences Center, New Orleans, Louisiana, USA
Nancy G Buccola
Center for Brain Science, Harvard University, Cambridge, Massachusetts, USA
Randy L Buckner
Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts, USA
Randy L Buckner & Joshua L Roffman
Athinoula A. Martinos Center, Massachusetts General Hospital, Boston, Massachusetts, USA
Randy L Buckner, Joshua L Roffman & Tracey L Petryshen
Department of Psychiatry, University of California at San Francisco, San Francisco, California, USA
William Byerley
Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, Utrecht, the Netherlands
Wiepke Cahn, René S Kahn, Eric Strengman & Roel A Ophoff
Department of Human Genetics, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Guiqing Cai & Joseph D Buxbaum
Schizophrenia Research Institute, Sydney, New South Wales, Australia
Murray J Cairns, Vaughan J Carr, Stanley V Catts, Frans A Henskens, Carmel M Loughland, Patricia T Michie, Christos Pantelis, Ulrich Schall, Rodney J Scott, Paul A Tooney, Jing Qin Wu & Assen V Jablensky
Priority Centre for Translational Neuroscience and Mental Health, University of Newcastle, Newcastle, New South Wales, Australia
Murray J Cairns, Frans A Henskens, Brian J Kelly, Carmel M Loughland, Ulrich Schall & Paul A Tooney
Centre Hospitalier du Rouvray and INSERM U1079, Faculty of Medicine, Rouen, France
Dominique Campion
Department of Human Genetics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA.,
Rita M Cantor & Roel A Ophoff
School of Psychiatry, University of New South Wales, Sydney, New South Wales, Australia
Vaughan J Carr
Department of Psychiatry, Royal Brisbane and Women's Hospital, University of Queensland, Brisbane, Queensland, Australia
Stanley V Catts
Department of Computer Science, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
Wei Cheng
Department of Psychiatry, Washington University, St. Louis, Missouri, USA
C Robert Cloninger & Dragan M Svrakic
Department of Child and Adolescent Psychiatry, Assistance Publique–Hospitaux de Paris, Pierre and Marie Curie Faculty of Medicine and Institute for Intelligent Systems and Robotics, Paris, France
David Cohen
Department of Psychiatry, Neuropsychiatric Genetics Research Group, Trinity College Dublin, Dublin, Ireland
Paul Cormican, Gary Donohoe, Derek W Morris, Aiden Corvin & Michael Gill
Instituto de Formación e Investigación Marqués de Valdecilla, University Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain
Benedicto Crespo-Facorro
Centro Investigación Biomédica en Red Salud Mental, Madrid, Spain
Benedicto Crespo-Facorro
Department of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
James J Crowley, Martilias S Farrell, Paola Giusti-Rodríguez, Yunjung Kim, Jin P Szatkiewicz, Stephanie Williams & Patrick F Sullivan
Department of Psychological Medicine, Queen Mary University of London, London, UK
David Curtis
Division of Psychiatry, Molecular Psychiatry Laboratory, University College London, London, UK
David Curtis, Jonathan Pimm, Hugh Gurling & Andrew McQuillin
Department of Psychiatry, Sheba Medical Center, Tel Hashomer, Israel
Michael Davidson & Mark Weiser
Institute of Human Genetics, University of Bonn, Bonn, Germany
Franziska Degenhardt, Andreas J Forstner, Stefan Herms, Per Hoffmann, Andrea Hofman, Sven Cichon & Markus M Nöthen
Department of Genomics, Life and Brain Center, Bonn, Germany
Franziska Degenhardt, Andreas J Forstner, Stefan Herms, Per Hoffmann, Andrea Hofman, Sven Cichon & Markus M Nöthen
VIB Department of Molecular Genetics, Applied Molecular Genomics Unit, University of Antwerp, Antwerp, Belgium
Jurgen Del Favero
Virginia Boston Health Care System, Brockton, Massachusetts, USA
Lynn E DeLisi & Robert W McCarley
Department of Psychiatry, Harvard Medical School, Boston, Massachusetts, USA
Lynn E DeLisi, Deborah L Levy, Robert W McCarley, Raquelle I Mesholam-Gately, Larry J Seidman & Tracey L Petryshen
First Department of Psychiatry, University of Athens Medical School, Athens, Greece
Dimitris Dikeos & George N Papadimitriou
Department of Psychiatry, University College Cork, Cork, Ireland
Timothy Dinan
Department of Medical Genetics, Oslo University Hospital, Oslo, Norway
Srdjan Djurovic
Cognitive Genetics and Therapy Group, School of Psychology and Discipline of Biochemistry, National University of Ireland Galway, Galway, Ireland
Gary Donohoe & Derek W Morris
Department of Psychiatry and Behavioral Sciences, NorthShore University HealthSystem, Evanston, Illinois, USA
Jubao Duan, Alan R Sanders & Pablo V Gejman
Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, Illinois, USA
Jubao Duan, Elliot S Gershon, Alan R Sanders & Pablo V Gejman
Department of Non-Communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK
Frank Dudbridge
Department of Psychiatry, University of Regensburg, Regensburg, Germany
Peter Eichhammer
Folkhälsan Research Center and Biomedicum Helsinki, Helsinki, Finland
Johan Eriksson
National Institute for Health and Welfare, Helsinki, Finland
Johan Eriksson & Veikko Salomaa
Department of General Practice, Helsinki University Central Hospital, University of Helsinki, Helsinki, Finland
Johan Eriksson
Translational Technologies and Bioinformatics, Pharma Research and Early Development, F. Hoffman-La Roche, Basel, Switzerland
Laurent Essioux
Mental Health Service Line, Washington Virginia Medical Center, Washington, DC, USA
Ayman H Fanous
Department of Psychiatry, Georgetown University, Washington, DC, USA
Ayman H Fanous
Department of Psychiatry, Virginia Commonwealth University, Richmond, Virginia, USA
Ayman H Fanous
Department of Psychiatry, Keck School of Medicine at the University of Southern California, Los Angeles, California, USA
Ayman H Fanous, James A Knowles, Michele T Pato & Carlos N Pato
Department of Genetic Epidemiology in Psychiatry, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Heidelberg, Germany
Josef Frank, Sandra Meier, Thomas G Schulze, Jana Strohmaier, Stephanie H Witt & Marcella Rietschel
Department of Genetics, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Lude Franke & Juha Karjalainen
Department of Psychiatry, University of Colorado Denver, Aurora, Colorado, USA
Robert Freedman & Ann Olincy
Center for Neurobehavioral Genetics, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, Los Angeles, California, USA.,
Nelson B Freimer & Roel A Ophoff
Division of Psychiatric Genomics, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Menachem Fromer, Shaun M Purcell, Panos Roussos, Douglas M Ruderfer, Eli A Stahl & Pamela Sklar
Psychiatric and Neurodevelopmental Genetics Unit, Massachusetts General Hospital, Boston, Massachusetts, USA
Menachem Fromer, Phil Lee, Jordan W Smoller, Aarno Palotie & Benjamin M Neale
Department of Human Genetics, University of Chicago, Chicago, Illinois, USA
Elliot S Gershon
Department of Psychiatry, University of Halle, Halle, Germany
Ina Giegling, Annette M Hartmann, Bettina Konte & Dan Rujescu
Department of Psychiatry, University of Munich, Munich, Germany
Ina Giegling & Dan Rujescu
Departments of Psychiatry, and Human and Molecular Genetics, INSERM, Institut de Myologie, Hôpital de la Pitiè-Salpêtrière, Paris, France
Stephanie Godard
Medical and Population Genetics Program, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA
Jacqueline I Goldstein, Joel N Hirschhorn, Hailiang Huang, Tune H Pers, Alkes Price, Eli A Stahl, Mark J Daly, Tõnu Esko & Benjamin M Neale
Queensland Brain Institute, University of Queensland, Brisbane, Queensland, Australia
Jacob Gratten, S Hong Lee, Peter M Visscher, Naomi R Wray & Bryan J Mowry
Department of Psychiatry, Academic Medical Centre, University of Amsterdam, Amsterdam, the Netherlands
Lieuwe de Haan & Carin J Meijer
Illumina, Inc., La Jolla, California, USA
Mark Hansen
J.J. Peters Virginia Medical Center, Bronx, New York, USA
Vahram Haroutunian
Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Vahram Haroutunian, Abraham Reichenberg, Joseph D Buxbaum & Pamela Sklar
School of Electrical Engineering and Computer Science, University of Newcastle, Newcastle, New South Wales, Australia
Frans A Henskens
Division of Medical Genetics, Department of Biomedicine, University of Basel, Basel, Switzerland
Stefan Herms, Per Hoffmann & Sven Cichon
Department of Genetics, Harvard Medical School, Boston, Massachusetts, USA
Joel N Hirschhorn, Tõnu Esko & Steven A McCarroll
Division of Endocrinology and Center for Basic and Translational Obesity Research, Boston Children's Hospital, Boston, Massachusetts, USA
Joel N Hirschhorn, Tune H Pers & Tõnu Esko
Department of Psychiatry, Fujita Health University School of Medicine, Toyoake, Japan
Masashi Ikeda & Nakao Iwata
Department of Psychiatry, Regional Centre for Clinical Research in Psychosis, Stavanger University Hospital, Stavanger, Norway
Inge Joa
Centre for Medical Research, University of Western Australia, Perth, Western Australia, Australia
Luba Kalaydjieva
Perkins Institute for Medical Research, University of Western Australia, Perth, Western Australia, Australia
Luba Kalaydjieva & Assen V Jablensky
Department of Psychology, University of Colorado Boulder, Boulder, Colorado, USA
Matthew C Keller
Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
James L Kennedy, Clement C Zai & Jo Knight
Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada
James L Kennedy, Clement C Zai & Jo Knight
Institute of Medical Science, University of Toronto, Toronto, Ontario, Canada
James L Kennedy & Jo Knight
Zilkha Neurogenetics Institute, Keck School of Medicine at the University of Southern California, Los Angeles, California, USA
James A Knowles, Michele T Pato & Carlos N Pato
Department of Child and Adolescent Psychiatry, Pierre and Marie Curie Faculty of Medicine, Paris, France
Claudine Laurent
Department of Psychiatry, Hadassah-Hebrew University Medical Center, Jerusalem, Israel
Bernard Lerer
Psychology Research Laboratory, McLean Hospital, Belmont, Massachusetts, USA
Deborah L Levy
Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland, USA
Kung-Yee Liang
Department of Psychiatry, Columbia University, New York, New York, USA
Jeffrey Lieberman & T Scott Stroup
Department of Mental Health and Substance Abuse Services, National Institute for Health and Welfare, Helsinki, Finland
Jouko Lönnqvist & Jaana Suvisaari
Department of Mental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA
Brion S Maher
Department of Psychiatry, University of Bonn, Bonn, Germany
Wolfgang Maier
CNRS, Laboratoire de Génétique Moléculaire de la Neurotransmission et des Processus Neurodégénératifs, Hôpital de la Pitié-Salpêtrière, Paris, France
Jacques Mallet
Department of Biomedicine, Aarhus University, Aarhus, Denmark
Manuel Mattheisen
Centre for Integrative Sequencing, iSEQ, Aarhus University, Aarhus, Denmark
Manuel Mattheisen
Department of Genomics Mathematics, University of Bonn, Bonn, Germany
Manuel Mattheisen
Research Unit, Sørlandet Hospital, Kristiansand, Norway
Morten Mattingsdal
Department of Psychiatry, National University of Ireland Galway, Galway, Ireland
Colm McDonald
Division of Psychiatry, University of Edinburgh, Edinburgh, UK
Andrew M McIntosh & Douglas H R Blackwood
Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, Edinburgh, UK
Andrew M McIntosh
Division of Mental Health and Addiction, Oslo University Hospital, Oslo, Norway
Ingrid Melle & Ole A Andreassen
Massachusetts Mental Health Center Public Psychiatry Division of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA
Raquelle I Mesholam-Gately & Larry J Seidman
Estonian Genome Center, University of Tartu, Tartu, Estonia
Andres Metspalu, Lili Milani & Tõnu Esko
School of Psychology, University of Newcastle, Newcastle, New South Wales, Australia
Patricia T Michie
First Psychiatric Clinic, Medical University, Sofia, Bulgaria
Vihra Milanova
Eli Lilly and Company, Ltd., Windlesham, UK
Younes Mokrab & David A Collier
Max Planck Institute of Psychiatry, Munich, Germany
Bertram Müller-Myhsok
Institute of Translational Medicine, University of Liverpool, Liverpool, UK
Bertram Müller-Myhsok
Cluster for Systems Neurology (SyNergy), Munich, Germany
Bertram Müller-Myhsok
Department of Psychiatry, Royal College of Surgeons in Ireland, Dublin, Ireland
Kieran C Murphy
Institute of Psychiatry, King's College London, London, UK
Robin M Murray & John Powell
Maastricht University Medical Centre, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht, the Netherlands
Inez Myin-Germeys & Jim Van Os
Department of Psychiatry and Psychotherapy, Jena University Hospital, Jena, Germany
Igor Nenadic
Queensland Centre for Mental Health Research, University of Queensland, Brisbane, Queensland, Australia
Deborah A Nertney & Bryan J Mowry
Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
Gerald Nestadt & Ann E Pulver
Department of Psychiatry, Trinity College Dublin, Dublin, Ireland
Kristin K Nicodemus
Eli Lilly and Company, Lilly Corporate Center, Indianapolis, Indiana, USA
Laura Nisenbaum
Department of Clinical Sciences, Psychiatry, Umeå University, Umeå, Sweden
Annelie Nordin & Rolf Adolfsson
DETECT Early Intervention Service for Psychosis, Blackrock, Ireland
Eadbhard O'Callaghan
Lawrence Berkeley National Laboratory, University of California at Berkeley, Berkeley, California, USA
Sang-Yun Oh
Centre for Public Health, Institute of Clinical Sciences, Queen's University Belfast, Belfast, UK
F Anthony O'Neill
Institute of Psychiatry, King's College London, London, UK
Jim Van Os
Melbourne Neuropsychiatry Centre, University of Melbourne and Melbourne Health, Melbourne, Victoria, Australia
Christos Pantelis
Public Health Genomics Unit, National Institute for Health and Welfare, Helsinki, Finland
Tiina Paunio & Olli Pietiläinen
Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
Diana O Perkins & Patrick F Sullivan
Department of Systems Biology, Center for Biological Sequence Analysis, Technical University of Denmark, Denmark
Tune H Pers
Institute for Molecular Medicine Finland, FIMM, University of Helsinki, Helsinki, Finland
Olli Pietiläinen & Aarno Palotie
Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts, USA
Alkes Price
Department of Psychiatry, University of Oxford, Oxford, UK
Digby Quested
Institute for Multiscale Biology, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Panos Roussos & Pamela Sklar
Neuroscience Therapeutic Area, Janssen Research and Development, Raritan, New Jersey, USA
Adam Savitz & Qingqin S Li
Department of Psychiatry and Psychotherapy, University of Göttingen, Göttingen, Germany
Thomas G Schulze
Psychiatry and Psychotherapy Clinic, University of Erlangen, Erlangen, Germany
Sibylle G Schwab
Hunter New England Health Service, Newcastle, New South Wales, Australia
Rodney J Scott
Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA
Jianxin Shi
Research and Development, Bronx Veterans Affairs Medical Center, New York, New York, USA
Jeremy M Silverman
Wellcome Trust Centre for Human Genetics, Oxford, UK
Chris C A Spencer
Department of Medical Genetics, University Medical Centre Utrecht, Utrecht, the Netherlands
Eric Strengman
Berkshire Healthcare NHS Foundation Trust, Bracknell, UK
Srinivas Thirumalai
Department of Psychiatry, University of Oulu, Oulu, Finland
Juha Veijola
Department of Psychiatry, University Hospital of Oulu, Oulu, Finland
Juha Veijola
Molecular and Cellular Therapeutics, Royal College of Surgeons in Ireland, Dublin, Ireland
John Waddington
Health Research Board, Dublin, Ireland
Dermot Walsh
School of Psychiatry and Clinical Neurosciences, University of Western Australia, Perth, Western Australia, Australia
Dieter B Wildenauer & Assen V Jablensky
Division of Psychiatry, University College London, London, UK
Elvira Bramon
Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, New York, USA
Joseph D Buxbaum
Institute of Neuroscience and Medicine (INM-1), Research Center Juelich, Juelich, Germany
Sven Cichon
Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, King's College London, London, UK
David A Collier
Department of Genetics, Hebrew University of Jerusalem, Jerusalem, Israel
Ariel Darvasi
Centre for Integrative Biology, University of Trento, Trento, Italy
Enrico Domenici
Centre for Clinical Research in Neuropsychiatry, School of Psychiatry and Clinical Neurosciences, University of Western Australia, Perth, Western Australia, Australia
Assen V Jablensky
Center for Human Genetic Research, Massachusetts General Hospital, Boston, Massachusetts, USA
Tracey L Petryshen
Department of Functional Genomics, Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University, Amsterdam, the Netherlands
Danielle Posthuma
Department of Complex Trait Genetics, Neuroscience Campus Amsterdam, VU University Medical Center Amsterdam, Amsterdam, the Netherlands
Danielle Posthuma
Department of Child and Adolescent Psychiatry, Erasmus University Medical Centre, Rotterdam, the Netherlands
Danielle Posthuma
Institute of Medical Sciences, University of Aberdeen, Aberdeen, UK
David St Clair
Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Thomas Werge
Department of Molecular Genetics and McLaughlin Centre, University of Toronto, Toronto, Ontario, Canada
Stephen W Scherer
Department of Cellular and Molecular Medicine, University of California, San Diego, La Jolla, California, USA.,
Jonathan Sebat
Management of the study, core analyses and content of the manuscript was the responsibility of the CNV Analysis Group, chaired by J. Sebat and jointly supervised by S.W.S. and B.M.N. together with the Schizophrenia Working Group, chaired by M.C.O'D. Core analyses were carried out by D.P.H., D. Merico, and C.R.M. Data Processing pipeline was implemented by C.R.M., B.T., W.W., D.S.G., M. Gujral, A. Shetty, and W.B. The A custom PGC CNV browser was developed by C.R.M., D.P.H. and B.T. Additional analyses and interpretations were contributed by W.W., D.A. and P.A.H. The individual studies or consortia contributing to the CNV meta-analysis were led by R.A., O.A.A., D.H.R.B., E. Bramon, J.D.B., A.C., D.A.C., S.C., A.D., E. Domenici, T.E., P.V.G., M.G., H.G., C.M.H., N.I., A.V.J., E.G.J., K.S.K., G.K., J. Knight, D.F.L., Q.S.L., J. Liu, S.A.M., A. McQuillin, J.L.M., B.J.M., M.M.N., M.C.O'D., R.A.O., M.J.O., A. Palotie, C.N.P., T.L.P., M.R., B.P.R., D.R., P. Sklar, D.S.C., P.F.S., J.T.R.W. and T.W. The remaining authors contributed to the recruitment, genotyping, or data processing for the contributing components of the meta-analysis. J. Sebat, B.M.N., M.C.O'D., C.R.M., D.P.H., and D. Merico drafted the manuscript, which was shaped by the management group. All other authors saw, had the opportunity to comment on and approved the final draft.