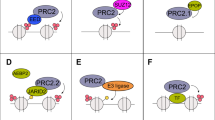
Abstract
Prostate cancer is driven by a combination of genetic and/or epigenetic alterations. Epigenetic alterations are frequently observed in all human cancers, yet how aberrant epigenetic signatures are established is poorly understood. Here we show that the gene encoding BAZ2A (TIP5), a factor previously implicated in epigenetic rRNA gene silencing, is overexpressed in prostate cancer and is paradoxically involved in maintaining prostate cancer cell growth, a feature specific to cancer cells. BAZ2A regulates numerous protein-coding genes and directly interacts with EZH2 to maintain epigenetic silencing at genes repressed in metastasis. BAZ2A overexpression is tightly associated with a molecular subtype displaying a CpG island methylator phenotype (CIMP). Finally, high BAZ2A levels serve as an independent predictor of biochemical recurrence in a cohort of 7,682 individuals with prostate cancer. This work identifies a new aberrant role for the epigenetic regulator BAZ2A, which can also serve as a useful marker for metastatic potential in prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Grasso, C.S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Weischenfeldt, J. et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell 23, 159–170 (2013).
Plass, C. et al. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nat. Rev. Genet. 14, 765–780 (2013).
Kim, J.H. et al. Deep sequencing reveals distinct patterns of DNA methylation in prostate cancer. Genome Res. 21, 1028–1041 (2011).
Kron, K. et al. Altered DNA methylation landscapes of polycomb-repressed loci are associated with prostate cancer progression and ERG oncogene expression in prostate cancer. Clin. Cancer Res. 19, 3450–3461 (2013).
Aryee, M.J. et al. DNA methylation alterations exhibit intraindividual stability and interindividual heterogeneity in prostate cancer metastases. Sci. Transl. Med. 5, 169ra10 (2013).
Varambally, S. et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002).
Bracken, A.P. et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 22, 5323–5335 (2003).
Hinz, S. et al. Expression of the polycomb group protein EZH2 and its relation to outcome in patients with urothelial carcinoma of the bladder. J. Cancer Res. Clin. Oncol. 134, 331–336 (2008).
Bachmann, I.M. et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J. Clin. Oncol. 24, 268–273 (2006).
Kleer, C.G. et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc. Natl. Acad. Sci. USA 100, 11606–11611 (2003).
Crea, F., Paolicchi, E., Marquez, V.E. & Danesi, R. Polycomb genes and cancer: time for clinical application? Crit. Rev. Oncol. Hematol. 83, 184–193 (2012).
Tsang, D.P. & Cheng, A.S. Epigenetic regulation of signaling pathways in cancer: role of the histone methyltransferase EZH2. J. Gastroenterol. Hepatol. 26, 19–27 (2011).
Richly, H., Aloia, L. & Di Croce, L. Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis. 2, e204 (2011).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Brase, J.C. et al. TMPRSS2-ERG–specific transcriptional modulation is associated with prostate cancer biomarkers and TGF-β signaling. BMC Cancer 11, 507 (2011).
Santoro, R., Li, J. & Grummt, I. The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nat. Genet. 32, 393–396 (2002).
Zhou, Y., Santoro, R. & Grummt, I. The chromatin remodeling complex NoRC targets HDAC1 to the ribosomal gene promoter and represses RNA polymerase I transcription. EMBO J. 21, 4632–4640 (2002).
Guetg, C., Scheifele, F., Rosenthal, F., Hottiger, M.O. & Santoro, R. Inheritance of silent rDNA chromatin is mediated by PARP1 via noncoding RNA. Mol. Cell 45, 790–800 (2012).
Hein, N., Hannan, K.M., George, A.J., Sanij, E. & Hannan, R.D. The nucleolus: an emerging target for cancer therapy. Trends Mol. Med. 19, 643–654 (2013).
Bywater, M.J. et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer–specific activation of p53. Cancer Cell 22, 51–65 (2012).
Cho, S. et al. MiRGator v3.0: a microRNA portal for deep sequencing, expression profiling and mRNA targeting. Nucleic Acids Res. 41, D252–D257 (2013).
Dong, Y.J. et al. MiR-133a inhibits colorectal cancer cell growth by direct targeting E3 ubiquitin ligase RFFL and activating p53-p21CIP1/WAF1 pathway. Gastroenterology 142, S185 (2012).
Uchida, Y. et al. MiR-133a induces apoptosis through direct regulation of GSTP1 in bladder cancer cell lines. Urol. Oncol. 31, 115–123 (2013).
Ji, F. et al. MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone 56, 220–226 (2013).
Tao, J. et al. MicroRNA-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol. Rep. 27, 1967–1975 (2012).
Kojima, S. et al. Tumor suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Eur. Urol. Suppl. 106, 405–413 (2012).
Li, J., Santoro, R., Koberna, K. & Grummt, I. The chromatin remodeling complex NoRC controls replication timing of rRNA genes. EMBO J. 24, 120–127 (2005).
Santoro, R., Lienemann, P. & Fussenegger, M. Epigenetic engineering of ribosomal RNA genes enhances protein production. PLoS ONE 4, e6653 (2009).
Guetg, C. et al. The NoRC complex mediates the heterochromatin formation and stability of silent rRNA genes and centromeric repeats. EMBO J. 29, 2135–2146 (2010).
Grummt, I. Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev. 17, 1691–1702 (2003).
Li, H., Chen, X., Calhoun-Davis, T., Claypool, K. & Tang, D.G. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 68, 1820–1825 (2008).
Sheng, X. et al. Isolation and enrichment of PC-3 prostate cancer stem-like cells using MACS and serum-free medium. Oncol. Lett. 5, 787–792 (2013).
Zhang, K. & Waxman, D.J. PC3 prostate tumor–initiating cells with molecular profile FAM65Bhigh/MFI2low/LEF1low increase tumor angiogenesis. Mol. Cancer 9, 319 (2010).
Doherty, R.E., Haywood-Small, S.L., Sisley, K. & Cross, N.A. Aldehyde dehydrogenase activity selects for the holoclone phenotype in prostate cancer cells. Biochem. Biophys. Res. Commun. 414, 801–807 (2011).
Collins, A.T., Berry, P.A., Hyde, C., Stower, M.J. & Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951 (2005).
Irshad, S. et al. A molecular signature predictive of indolent prostate cancer. Sci. Transl. Med. 5, 202ra122 (2013).
Yu, Y.P. et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J. Clin. Oncol. 22, 2790–2799 (2004).
Wang, Y. et al. Survey of differentially methylated promoters in prostate cancer cell lines. Neoplasia 7, 748–760 (2005).
Mori, Y. et al. Novel candidate colorectal cancer biomarkers identified by methylation microarray-based scanning. Endocr. Relat. Cancer 18, 465–478 (2011).
Kinoshita, M., Nakagawa, T., Shimizu, A. & Katsuoka, Y. Differently regulated androgen receptor transcriptional complex in prostate cancer compared with normal prostate. Int. J. Urol. 12, 390–397 (2005).
Novak, P. et al. Epigenetic inactivation of the HOXA gene cluster in breast cancer. Cancer Res. 66, 10664–10670 (2006).
Zhang, J.S., Gong, A. & Young, C.Y. ZNF185, an actin-cytoskeleton-associated growth inhibitory LIM protein in prostate cancer. Oncogene 26, 111–122 (2007).
Cheng, X.F. et al. Growth inhibitory effect of Kruppel-like factor 6 on human prostatic carcinoma and renal carcinoma cell lines. Tohoku J. Exp. Med. 216, 35–45 (2008).
Wang, N. et al. Screening and identification of distant metastasis-related differentially expressed genes in human squamous cell lung carcinoma. Anat. Rec. (Hoboken) 295, 748–757 (2012).
Maksimovic, J., Gordon, L. & Oshlack, A. SWAN: subset-quantile within array normalization for Illumina Infinium HumanMethylation450 BeadChips. Genome Biol. 13, R44 (2012).
Lee, W.H. et al. Cytidine methylation of regulatory sequences near the π-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc. Natl. Acad. Sci. USA 91, 11733–11737 (1994).
Yu, J. et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 (2010).
Issa, J.P. CpG island methylator phenotype in cancer. Nat. Rev. Cancer 4, 988–993 (2004).
Zouridis, H. et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci. Transl. Med. 4, 156ra140 (2012).
McCabe, M.T., Lee, E.K. & Vertino, P.M. A multifactorial signature of DNA sequence and polycomb binding predicts aberrant CpG island methylation. Cancer Res. 69, 282–291 (2009).
Cho, N.Y. et al. Hypermethylation of CpG island loci and hypomethylation of LINE-1 and Alu repeats in prostate adenocarcinoma and their relationship to clinicopathological features. J. Pathol. 211, 269–277 (2007).
Shyr, C.R. et al. Tumor suppressor PAX6 functions as androgen receptor co-repressor to inhibit prostate cancer growth. Prostate 70, 190–199 (2010).
Nguyen, A.H. et al. Gata3 antagonizes cancer progression in Pten-deficient prostates. Hum. Mol. Genet. 22, 2400–2410 (2013).
Kypta, R.M. & Waxman, J. Wnt/β-catenin signalling in prostate cancer. Nat. Rev. Urol. 9, 418–428 (2012).
Kashat, M. et al. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am. J. Transl. Res. 4, 432–442 (2012).
Östling, P. et al. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 71, 1956–1967 (2011).
Shi, X.B. et al. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene 32, 4130–4138 (2013).
Dawson, M.A., Kouzarides, T. & Huntly, B.J. Targeting epigenetic readers in cancer. N. Engl. J. Med. 367, 647–657 (2012).
Zhou, Y. & Grummt, I. The PHD finger/bromodomain of NoRC interacts with acetylated histone H4K16 and is sufficient for rDNA silencing. Curr. Biol. 15, 1434–1438 (2005).
Li, B. & Dewey, C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Metzig, M. et al. An RNAi screen identifies USP2 as a factor required for TNF-α–induced NF-κB signaling. Int. J. Cancer 129, 607–618 (2011).
Santoro, R. Analysis of chromatin composition of repetitive sequences: the ChIP-Chop assay. Methods Mol. Biol. 1094, 319–328 (2014).
Subramanian, A., Kuehn, H., Gould, J., Tamayo, P. & Mesirov, J.P. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics 23, 3251–3253 (2007).
Schlomm, T. et al. Clinical significance of p53 alterations in surgically treated prostate cancers. Mod. Pathol. 21, 1371–1378 (2008).
Mirlacher, M. & Simon, R. Recipient block TMA technique. Methods Mol. Biol. 664, 37–44 (2010).
Minner, S. et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate 71, 281–288 (2011).
Grupp, K. et al. Cysteine-rich secretory protein 3 overexpression is linked to a subset of PTEN-deleted ERG fusion–positive prostate cancers with early biochemical recurrence. Mod. Pathol. 26, 733–742 (2013).
Muller, J. et al. Loss of pSer2448-mTOR expression is linked to adverse prognosis and tumor progression in ERG-fusion-positive cancers. Int. J. Cancer 132, 1333–1340 (2013).
Acknowledgements
We acknowledge the entire team of the German ICGC Project on Early Onset Prostate Cancer. We thank M. Lupien, C. Schmidt, D. Wuttig, O. Bogatyrova, A. Postępska-Igielska and N. Schmitt for assistance with experiments and data. This project was supported by the German Federal Ministry of Education and Science in the Program for Medical Genome Research including the EOPC project within ICGC (FKZ; 01KU1001A, 01KU1001B, 01KU1001C, 01KU1001D and 01GS0890), by Krebsforschung Schweiz (KFS; 02732-02-2011), by the Swiss National Science Foundation (SNF; 310003A-135801 and 31003A-152854), by Swiss Life, by a Müller Molecular Life Science fellowship and by Mäxi Stiftung. We acknowledge assistance provided by the Genomics and Proteomics Core Facility at the German Cancer Research Center. In particular, we acknowledge the excellent technical support of M. Schick.
Author information
Authors and Affiliations
Consortia
Contributions
L.G., S.C.F., C.C.O., R. Simon, K.G., C.Y.G., D.B., M.P., C.B., M.W. and R.K. designed the experiments and performed experimental work. L.G., R.E., C.C.O., R. Simon, Z.G., R.K., M.D.R., M.S. and K.G. performed data analysis. R.K., G.S. and H.S. provided clinical samples or data. L.G., S.C.F., C.C.O., R. Simon, C.P., G.S., R.E. and R. Santoro prepared the manuscript and figures. M.-L.Y., B.B., J.K., T.S., G.S., R.E., H.S., C.P. and R. Santoro provided project leadership. All authors contributed to the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A list of contributing members and affiliations appears in the Supplementary Note.
Integrated supplementary information
Supplementary Figure 1 Overexpression of BAZ2A via downregulation of miR-133a influences cell proliferation in prostate cancer.
Related to Figure 1. (a) Related to Figure 1b. Expression profiles of the upstream binding factor UBF from data sets GSE3325 and GSE6919. (b) Related to Figure 1d. Luciferase assays evaluating the direct interaction of miRNAs with the BAZ2A 3′ UTR. miR-133a specifically interacts with a distal conserved site in the 3′ UTR, while another miRNA, miR-145, although predicted to target the 3′ UTR, does not interact. (c) Related to Figure 1e. Overexpression of miR-133a results in the downregulation of BAZ2A in the BPH1 and DU145 prostate cell lines in comparison to other miRNAs, miR-139 and miR-145, used as negative controls. Expression was measured by three independent primer-probe pairs relative to the average of GAPDH, ACTB and HPRT. Each experiment was performed in triplicate for each cell line. (d) Related to Figure 1f. qRT-PCR showing the efficiency of BAZ2A knockdown of the indicated cell lines after treatment with siRNA-BAZ2A. (e) Related to Figure 1f. qRT-PCR showing 45S pre-rRNA transcript levels for the indicated cells after treatment with siRNA-BAZ2A. Data represent the average of three to four independent experiments. (f) Related to Figure 1f. Cell viability measured by WST1 assay of PC3 and RWPE1 cells treated with siRNA-BAZ2A. Data represent the average of two independent experiments. (g) Related to Figure 1g. WST1 assay of PC3 and RWPE1 cells treated with siRNA-EZH2. Data represent the average of two independent experiments.
Supplementary Figure 2 Interrogation of the rate of genetic alterations in the BAZ2A locus.
Related to Figure 1. Histogram showing the frequency of mutations and copy number alterations in the BAZ2A locus using all publically available TCGA data sets, including 69 cancer studies obtained from the cBio database. Highlighted are the five prostate cancer studies, which show an overall lack of genetic aberrations. Only 1 case showed amplification in a total of 514 prostate adenocarcinoma samples investigated, and 0 cases showed aberrations in 61 metastatic samples (green bars, mutation; red bars, amplification; blue bars, deletion; gray bars, multiple deletions).
Supplementary Figure 3 BAZ2A influences the cell cycle progression of prostate cancer cells.
Related to Figure 2. (a) Depletion of BAZ2A does not induce apoptosis. PC3 and RWPE1 cells were labeled with Annexin V–AlexaFluor 647 and propidium iodide. (b) Depletion of BAZ2A does not induce senescence. In vitro senescence analysis was performed by measuring β-gal activity in PC3 cells depleted of BAZ2A by siRNA. Treatment with doxorubin served as a positive control for staining. (c) Flow cytometry analysis of PC3 and RWPE1 cells depleted of BAZ2A by siRNA treatment. Images are representative of two independent experiments. (d) Flow cytometry analysis of PC3 and RWPE1 cells synchronized at G1/S and at the indicated time points after release into S phase. Images are representative of two independent experiments. (e) Expression of CDKN1A is upregulated upon knockdown of BAZ2A in PC3 cells. Top, qRT-PCR showing BAZ2A mRNA levels. Upregulation of CDKN1A was also consistent with microarray data for PC3 cells (log2 fold change = 0.66, P = 0.005 upon siRNA-BAZ2A treatment; log2 fold change = 0.84, P = 0.001 upon siRNA-EZH2 treatment; Supplementary Table 3). No changes were detected in RWPE1 cells upon siRNA-BAZ2A treatment (Supplementary Table 4). Bottom, CDKN1A immunoblots of PC3 cells depleted of BAZ2A by siRNA. (f) Related to Figure 2f. qRT-PCR showing reduction in BAZ2A expression in PC3 cell lines stably expressing shRNA-BAZ2A. Data were normalized to GAPDH mRNA levels. (g) Related to Figure 2f. Clonogenic assay of PC3 cells expressing shRNA-BAZ2A. Cells (500) were cultured in a 6-well plate for 8 d. Crystal violet staining showed the presence of dense (shRNA-Control) and dispersed (shRNA-BAZ2A) cell colonies. Images are representative of three independent experiments.
Supplementary Figure 4 A nucleoplasmatic BAZ2A fraction characterizes PC3 and RWPE1 cells.
Related to Figure 3. Immunofluorescence analysis of BAZ2A in mouse NIH3T3 cells and human PC3 and RWPE1 cells. Signal for UBF was used to visualize nucleoli. Whereas in mouse fibroblast cells BAZ2A is mainly localized within nucleoli, both PC3 and RWPE1 cells show an abundant nucleoplasmatic fraction. The specificity of the antibody to BAZ2A was verified by the lack of signal in PC3 cells depleted of BAZ2A by siRNA-BAZ2A treatment.
Supplementary Figure 5 Gene set enrichment analysis following knockdown of BAZ2A or EZH2 in PC3 cells.
Related to Figure 3. Significant gene sets were chosen for display to highlight the regulation of similar prostate cancer–relevant pathways by BAZ2A and EZH2. For a complete list of significant gene sets, see Supplementary Table 5.
Supplementary Figure 6 Genes regulated by BAZ2A and EZH2 in both PC3 and RWPE1 cells do not act on relevant common biological processes.
Related to Figure 3. (a) Venn diagrams showing genes regulated in both PC3 and RWPE1 cells upon knockdown of BAZ2A and EZH2. (b) Diagrams showing the effects on expression of genes regulated by BAZ2A, EZH2 or both BAZ2A and EZH2 in PC3 and RWPE1 cells. (c) Top six biological process gene ontology (GO) terms as determined using the Database for Annotation, Visualization and Integrated Discovery (DAVID) for genes up- and downregulated upon BAZ2A and EZH2 knockdown in PC3 and RWPE1 cells. The data determined that genes common in PC3 and RWPE1 cells do not display any relevant biological pathways.
Supplementary Figure 7 BAZ2A- and EZH2-regulated genes show a substantial overlap of biological pathways in PC3 but not in RWPE1 cells.
Related to Figure 3. The top seven biological process gene ontology (GO) terms in PC3 and RWPE1 cells as determined using DAVID.
Supplementary Figure 8 BAZ2A and EZH2 coordinate gene repression in prostate cancer cells.
Related to Figure 4. (a) Related to Figure 3d. Knockdown efficiency of BAZ2A and EZH2 in PC3 and RWPE1 cells. Data were normalized to GAPDH mRNA levels. (b) Related to Figure 4d. Knockdown efficiency of BAZ2A and EZH2 in PC3 cells. Data were normalized to GAPDH mRNA levels. (c) Related to Figure 4b, e. Specificity of the described ChIP assay. The association of BAZ2A with the rDNA promoter is greater than with the rDNA coding region. EZH2 does not associate with α-satellites. Data are normalized to input and to association with the rDNA promoter (left) and the HOXA7 promoter (right) in PC3 cells treated with siRNA-Control.
Supplementary Figure 9 Levels of DNA methylation found at the promoters of prostate cancer–relevant genes selected from 450k analysis.
Related to Figure 5. (a) GSTP1 and APC are consistently hypermethylated in all prostate tumor samples relative to normal samples, demonstrating that tumor content does not appreciably differ among tumor samples. (b) Hypermethylation of the known tumor suppressors WT1 (Int. J. Oncol. 24, 461–471, 2004), PAX6 (ref.), GATA3 (ref.) and SFRP2 (ref.) occurs primarily in the CIMP+/BAZ2A-high subtype. (c) Hypermethylation of miRNAs 9-1, 9-3, 124-2, 34b and 34c, known to regulate androgen receptor expression, also occurs primarily in the CIMP+/BAZ2A-high subtype.
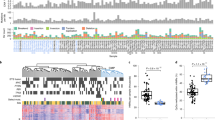
Supplementary Figure 10 Validation of global DNA methylation subtypes and association with BAZ2A expression in TCGA data.
Related to Figure 5. 450k DNA methylation prostate cancer profiles downloaded from the TCGA database were separated into the tumors with the 20 highest and lowest BAZ2A expression levels. Cluster analysis (using Euclidean distance measurement) of the top 3,000 most variable CpGs reveals 2 molecular subgroups, 1 that is similar to normal samples (normal-like subgroup) and 1 displaying profound hypermethylation (CIMP subgroup). High BAZ2A expression is associated with the CIMP subgroup. The heat map to the right shows normal prostate samples.
Supplementary Figure 11 Gene set enrichment analysis in BAZ2A-high tumors.
Related to Figure 5. Gene set enrichment analysis for prostate cancer–relevant pathways and gene ontology terms found to be significantly overrepresented (FDR q < 0.05) in BAZ2A-high/CIMP-like versus BAZ2A-low/normal-like tumors.
Supplementary Figure 12 Gene ontology analysis in BAZ2A-high tumors.
Related to Figure 5. Top seven biological process gene ontology (GO) terms of genes with hypermethylated promoters in BAZ2A-high versus BAZ2A-low tumors as determined using DAVID.
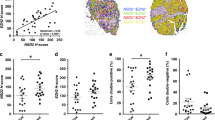
Supplementary Figure 13 BAZ2A staining of prostate tumor specimens.
Related to Figure 6. Wide-field micrographs of representative prostate tumor specimens showing positive (a) and negative (b) BAZ2A staining.
Supplementary Figure 14 PSA recurrence-free survival.
Related to Figure 6. PSA recurrence-free survival in (a) ERG-negative, (b) ERG-positive, (c) Gleason grade ≤3+3 (indolent prostate cancer) and (d) Gleason grade ≥4+4 (high-risk) patients.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14, Supplementary Tables 2, 5 and 8–13, and Supplementary Note. (PDF 8750 kb)
Supplementary Table 1
Expression of epigenetic regulators in prostate tumors. Related to Figure 1a. (a) List of 709 genes associated with epigenetic regulation3. (b) Expression differences of epigenetic regulators between normal prostate and prostate tumor samples. (XLSX 42 kb)
Supplementary Table 3
Gene expression analysis of PC3 cells upon BAZ2A or EZH2 knockdown. Related to Figure 3. Genes upregulated or downregulated by siRNA-BAZ2A, siRNA-EZH2 or both are shown. The list of RBEPM genes is included in the list of genes upregulated by BAZ2A or EZH2 depletion. (XLSX 591 kb)
Supplementary Table 4
Gene expression analysis of RWPE1 cells upon BAZ2A or EZH2 knockdown. Related to Figure 3. Genes upregulated or downregulated by siRNA-BAZ2A, siRNA-EZH2 or both are shown. (XLSX 537 kb)
Supplementary Table 6
Genes regulated in both PC3 and RWPE1 cells upon BAZ2A or EZH2 knockdown. Related to Figure 3. (XLSX 111 kb)
Supplementary Table 7
List of differentially methylated genes in BAZ2A-high versus BAZ2A-low tumors. Related to Figure 5. (XLSX 608 kb)
Rights and permissions
About this article
Cite this article
Gu, L., Frommel, S., Oakes, C. et al. BAZ2A (TIP5) is involved in epigenetic alterations in prostate cancer and its overexpression predicts disease recurrence. Nat Genet 47, 22–30 (2015). https://doi.org/10.1038/ng.3165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3165
This article is cited by
-
A new prediction model of hepatocellular carcinoma based on N7-methylguanosine modification
BMC Gastroenterology (2023)
-
LINC00885 promotes cervical cancer progression through sponging miR-3150b-3p and upregulating BAZ2A
Biology Direct (2022)
-
The emerging role of ISWI chromatin remodeling complexes in cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Proteomic analysis of the effects of antler extract on chondrocyte proliferation, differentiation and apoptosis
Molecular Biology Reports (2019)
-
Epidermal Wnt signalling regulates transcriptome heterogeneity and proliferative fate in neighbouring cells
Genome Biology (2018)