Abstract
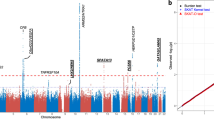
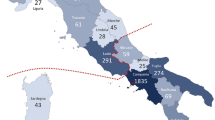
Macular degeneration is a common cause of blindness in the elderly. To identify rare coding variants associated with a large increase in risk of age-related macular degeneration (AMD), we sequenced 2,335 cases and 789 controls in 10 candidate loci (57 genes). To increase power, we augmented our control set with ancestry-matched exome-sequenced controls. An analysis of coding variation in 2,268 AMD cases and 2,268 ancestry-matched controls identified 2 large-effect rare variants: previously described p.Arg1210Cys encoded in the CFH gene (case frequency (fcase) = 0.51%; control frequency (fcontrol) = 0.02%; odds ratio (OR) = 23.11) and newly identified p.Lys155Gln encoded in the C3 gene (fcase = 1.06%; fcontrol = 0.39%; OR = 2.68). The variants suggest decreased inhibition of C3 by complement factor H, resulting in increased activation of the alternative complement pathway, as a key component of disease biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Accession codes
References
Priya, R.R., Chew, E.Y. & Swaroop, A. Genetic studies of age-related macular degeneration: lessons, challenges, and opportunities for disease management. Ophthalmology 119, 2526–2536 (2012).
Swaroop, A., Chew, E.Y., Rickman, C.B. & Abecasis, G.R. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu. Rev. Genomics Hum. Genet. 10, 19–43 (2009).
Friedman, D.S. et al. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 122, 564–572 (2004).
Haines, J.L. et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419–421 (2005).
Edwards, A.O. et al. Complement factor H polymorphism and age-related macular degeneration. Science 308, 421–424 (2005).
Klein, R.J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389 (2005).
Jakobsdottir, J. et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am. J. Hum. Genet. 77, 389–407 (2005).
Rivera, A. et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum. Mol. Genet. 14, 3227–3236 (2005).
Yates, J.R. et al. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 357, 553–561 (2007).
Gold, B. et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 38, 458–462 (2006).
Fagerness, J.A. et al. Variation near complement factor I is associated with risk of advanced AMD. Eur. J. Hum. Genet. 17, 100–104 (2009).
Maller, J.B. et al. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat. Genet. 39, 1200–1201 (2007).
Fritsche, L.G. et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 45, 433–439 (2013).
Arakawa, S. et al. Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population. Nat. Genet. 43, 1001–1004 (2011).
Nejentsev, S., Walker, N., Riches, D., Egholm, M. & Todd, J.A. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324, 387–389 (2009).
Raychaudhuri, S. et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat. Genet. 43, 1232–1236 (2011).
Józsi, M. et al. Factor H and atypical hemolytic uremic syndrome: mutations in the C-terminus cause structural changes and defective recognition functions. J. Am. Soc. Nephrol. 17, 170–177 (2006).
Manuelian, T. et al. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J. Clin. Invest. 111, 1181–1190 (2003).
Ferreira, V.P. et al. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J. Immunol. 182, 7009–7018 (2009).
Chen, W. et al. Genetic variants near TIMP3 and high-density lipoprotein–associated loci influence susceptibility to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 107, 7401–7406 (2010).
Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 107, 2224–2232 (2000).
Tennessen, J.A. et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 337, 64–69 (2012).
Fu, W. et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493, 216–220 (2013).
1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Mathieson, I. & McVean, G. Differential confounding of rare and common variants in spatially structured populations. Nat. Genet. 44, 243–246 (2012).
Li, J.Z. et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science 319, 1100–1104 (2008).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Li, B. & Leal, S.M. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am. J. Hum. Genet. 83, 311–321 (2008).
Price, A.L. et al. Pooled association tests for rare variants in exon-resequencing studies. Am. J. Hum. Genet. 86, 832–838 (2010).
Wu, M.C. et al. Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 89, 82–93 (2011).
Helgason, H. et al. A rare nonsynonymous sequence variant in C3 confers high risk of age-related macular degeneration. Nat. Genet. 10.1038/ng.2740 (15 September 2013).
AREDS2 Research Group. et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 119, 2282–2289 (2012).
Heurich, M. et al. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl. Acad. Sci. USA 108, 8761–8766 (2011).
Fritsche, L.G. et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat. Genet. 40, 892–896 (2008).
Kanda, A. et al. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc. Natl. Acad. Sci. USA 104, 16227–16232 (2007).
Dewan, A. et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 314, 989–992 (2006).
Sánchez-Corral, P. et al. Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am. J. Hum. Genet. 71, 1285–1295 (2002).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Jun, G. et al. Detecting and estimating contamination of human DNA samples in sequencing and array-based genotype data. Am. J. Hum. Genet. 91, 839–848 (2012).
Cox, D.R. & Shell, E.J. Analysis of Binary Data 2nd edn. (CRC Press, New York, 1989).
Hirji, K.F., Mehta, C.R. & Patel, N.R. Computing distributions for exact logistic-regression. J. Am. Stat. Assoc. 82, 1110–1117 (1987).
Rosenbaum, P.R. & Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 70, 41–55 (1983).
Acknowledgements
We thank all study participants for their generous volunteering. We thank B. Li, W. Chen, C. Sidore, T. Teslovich, L. Fritsche and M. Boehnke for useful discussion and suggestions. This project was supported by grants from the US National Institutes of Health (National Eye Institute, National Human Genome Research Institute; grants EY022005, HG007022, HG005552, EY016862, U54HG003079 and EY09859); the Medical Research Council, UK (grant G0000067); the Deutsche Forschungsgemeinschaft (grant WE1259/19-2); the Alcon Research Institute; The UK Department of Health's National Institute for Health Research (NIHR) Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and the UCL Institute of Ophthalmology; Research to Prevent Blindness (New York); the Thome Memorial Foundation; the Harold and Pauline Price Foundation; and the National Health and Medical Research Council of Australia (NHMRC) Clinical Research Excellence (grant 529923, NHMRC practitioner fellowship 529905 and NHMRC Senior Research Fellowship 1028444). The study was also supported by the Intramural Research Program (Computational Medicine Initiative) of the National Eye Institute. The Centre for Eye Research Australia (CERA) receives operational infrastructure support from the Victorian Government. The views expressed in the publication are those of the authors and not necessarily those of their employers or the funders.
Author information
Authors and Affiliations
Contributions
R.K.W., J.R.H., E.Y.C., D.S., E.R.M., A.S. and G.R.A. conceived, designed and supervised the experiments. X.Z. and G.R.A. wrote the initial version of the manuscript. X.Z., D.E.L., C.W. and D.C.K. analyzed the data. D.E.L., D.C.K., R.S.F., L.L.F. and C.C.F. supervised data generation. C.W. developed statistical methodology. Y.V.S. analyzed protein structures. K.E.B. supervised sample and data collection. J.B.-G., G.J., Y.H., H.M.K. and D.L. contributed data and analysis tools. M.B., R.R. and A.B. assisted in laboratory experiments. M.O. and F.G. carried out experimental studies (genotyping and data analysis) for the Michigan and Regensburg samples, respectively. C.v.S. recruited the family members of sporadic AMD cases and controls and collected peripheral blood samples for the Regensburg study. L.M.O., M.A.P.-V. and J.L.H. provided results and analysis for the Vanderbilt/Miami samples. G.H.S.B., A.H., C.M.v.D. and C.C.W.K. provided results and analysis for samples from the Rotterdam Study, Erasmus Medical Center. V.C., A.T.M., H.S. and J.R.W.Y. provided results and analysis for the Cambridge AMD Study samples. Y.J., Y.P.C., D.E.W. and M.B.G. provided results and analysis for the University of California, Los Angeles/University of Pittsburgh samples. D.J.M., I.K.K., L.A.F. and M.M.D. provided results and analysis for the Utah samples. M.P.J., J.B. and M.L.K. provided results and analysis for the Oregon Health Sciences Center samples. S.C., A.J.R., R.H.G. and P.N.B. provided results and analysis for the University of Melbourne samples. H.L., H.O., M.M.Z. and K.Z. provided results and analysis for the University of California, San Diego samples. C.L. and F.G.P. provided results and analysis for a cohort of individuals with aHUS. B.H.F.W. was involved in the design and planning of the Southern Germany AMD Study. B.H.F.W. participated in study coordination and critically read the manuscript. All authors have critically commented on this manuscript.
Corresponding author
Ethics declarations
Competing interests
G.R.A., X.Z., C.W. and A.S. are potential beneficiaries of a University of Michigan patent that is now pending that describes association between variant p.Lys155Gln encoded by the complement 3 gene and AMD.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–6 (PDF 1553 kb)
Rights and permissions
About this article
Cite this article
Zhan, X., Larson, D., Wang, C. et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat Genet 45, 1375–1379 (2013). https://doi.org/10.1038/ng.2758
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2758
This article is cited by
-
Age-related macular degeneration and premorbid allergic diseases: a population-based case–control study
Scientific Reports (2021)
-
A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration
Nature Communications (2019)