Abstract
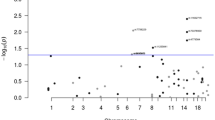
To identify new genetic factors for colorectal cancer (CRC), we conducted a genome-wide association study in east Asians. By analyzing genome-wide data in 2,098 cases and 5,749 controls, we selected 64 promising SNPs for replication in an independent set of samples, including up to 5,358 cases and 5,922 controls. We identified four SNPs with association P values of 8.58 × 10−7 to 3.77 × 10−10 in the combined analysis of all east Asian samples. Three of the four were replicated in a study conducted in 26,060 individuals of European descent, with combined P values of 1.22 × 10−10 for rs647161 (5q31.1), 6.64 × 10−9 for rs2423279 (20p12.3) and 3.06 × 10−8 for rs10774214 (12p13.32 near the CCND2 gene), derived from meta-analysis of data from both east Asian and European-ancestry populations. This study identified three new CRC susceptibility loci and provides additional insight into the genetics and biology of CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
de la Chapelle, A. Genetic predisposition to colorectal cancer. Nat. Rev. Cancer 4, 769–780 (2004).
Dong, L.M. et al. Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. J. Am. Med. Assoc. 299, 2423–2436 (2008).
Zanke, B.W. et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat. Genet. 39, 989–994 (2007).
Tomlinson, I. et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat. Genet. 39, 984–988 (2007).
Broderick, P. et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat. Genet. 39, 1315–1317 (2007).
Jaeger, E. et al. Common genetic variants at the CRAC1 (HMPS) locus on chromosome 15q13.3 influence colorectal cancer risk. Nat. Genet. 40, 26–28 (2008).
Tenesa, A. et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat. Genet. 40, 631–637 (2008).
Tomlinson, I.P. et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat. Genet. 40, 623–630 (2008).
Houlston, R.S. et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat. Genet. 40, 1426–1435 (2008).
Houlston, R.S. et al. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nat. Genet. 42, 973–977 (2010).
Cui, R. et al. Common variant in 6q26-q27 is associated with distal colon cancer in an Asian population. Gut 60, 799–805 (2011).
He, J. et al. Generalizability and epidemiologic characterization of eleven colorectal cancer GWAS hits in multiple populations. Cancer Epidemiol. Biomarkers Prev. 20, 70–81 (2011).
Zheng, W. et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat. Genet. 41, 324–328 (2009).
Bei, J.X. et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat. Genet. 42, 599–603 (2010).
Jee, S.H. et al. Adiponectin concentrations: a genome-wide association study. Am. J. Hum. Genet. 87, 545–552 (2010).
Nakata, I. et al. Association between the SERPING1 gene and age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese. PLoS ONE 6, e19108 (2011).
Li, Y., Willer, C.J., Ding, J., Scheet, P. & Abecasis, G.R. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 34, 816–834 (2010).
Willer, C.J., Li, Y. & Abecasis, G.R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Peters, U. et al. Meta-analysis of new genome-wide association studies of colorectal cancer risk. Hum. Genet. 131, 217–234 (2012).
Figueiredo, J.C. et al. Genotype-environment interactions in microsatellite stable/microsatellite instability–low colorectal cancer: results from a genome-wide association study. Cancer Epidemiol. Biomarkers Prev. 20, 758–766 (2011).
Musgrove, E.A., Caldon, C.E., Barraclough, J., Stone, A. & Sutherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 11, 558–572 (2011).
Mermelshtein, A. et al. Expression of D-type cyclins in colon cancer and in cell lines from colon carcinomas. Br. J. Cancer 93, 338–345 (2005).
Sarkar, R. et al. Expression of cyclin D2 is an independent predictor of the development of hepatic metastasis in colorectal cancer. Colorectal Dis. 12, 316–323 (2010).
Gundem, G. et al. IntOGen: integration and data mining of multidimensional oncogenomic data. Nat. Methods 7, 92–93 (2010).
Matys, V. et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 34, D108–D110 (2006).
Kolfschoten, I.G. et al. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell 121, 849–858 (2005).
Chen, Y. et al. Decreased PITX1 homeobox gene expression in human lung cancer. Lung Cancer 55, 287–294 (2007).
Chen, Y.N., Chen, H., Xu, Y., Zhang, X. & Luo, Y. Expression of pituitary homeobox 1 gene in human gastric carcinogenesis and its clinicopathological significance. World J. Gastroenterol. 14, 292–297 (2008).
Lord, R.V. et al. Increased CDX2 and decreased PITX1 homeobox gene expression in Barrett's esophagus and Barrett's-associated adenocarcinoma. Surgery 138, 924–931 (2005).
Nagel, S. et al. Activation of paired-homeobox gene PITX1 by del(5)(q31) in T-cell acute lymphoblastic leukemia. Leuk. Lymphoma 52, 1348–1359 (2011).
Watanabe, T. et al. Differential gene expression signatures between colorectal cancers with and without KRAS mutations: crosstalk between the KRAS pathway and other signalling pathways. Eur. J. Cancer 47, 1946–1954 (2011).
Liu, D.X. & Lobie, P.E. Transcriptional activation of p53 by Pitx1. Cell Death Differ. 14, 1893–1907 (2007).
Qi, D.L. et al. Identification of PITX1 as a TERT suppressor gene located on human chromosome 5. Mol. Cell Biol. 31, 1624–1636 (2011).
Knösel, T. et al. Loss of desmocollin 1-3 and homeobox genes PITX1 and CDX2 are associated with tumor progression and survival in colorectal carcinoma. Int. J. Colorectal Dis. 27, 1391–1399 (2012).
Zeller, T. et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS ONE 5, e10693 (2010).
Long, J. et al. Genome-wide association study in east Asians identifies novel susceptibility loci for breast cancer. PLoS Genet. 8, e1002532 (2012).
Shu, X.O. et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 6, pii: e1001127 (2010).
Zheng, W. et al. Genetic and clinical predictors for breast cancer risk assessment and stratification among Chinese women. J. Natl. Cancer Inst. 102, 972–981 (2010).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Sinnott, J.A. & Kraft, P. Artifact due to differential error when cases and controls are imputed from different platforms. Hum. Genet. 131, 111–119 (2012).
Jiao, S., Hsu, L., Hutter, C.M. & Peters, U. The use of imputed values in the meta-analysis of genome-wide association studies. Genet. Epidemiol. 35, 597–605 (2011).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Lau, J., Ioannidis, J.P. & Schmid, C.H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 127, 820–826 (1997).
Higgins, J.P. & Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Zhang, B., Beeghly-Fadiel, A., Long, J. & Zheng, W. Genetic variants associated with breast-cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence. Lancet Oncol. 12, 477–488 (2011).
Barrett, J.C., Fry, B., Maller, J. & Daly, M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Pruim, R.J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).
Acknowledgements
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The authors wish to thank the study participants and research staff for their contributions and commitment to this project, R. Courtney for DNA preparation, J. He for data processing and analyses, and M.J. Daly for clerical support in manuscript preparation. This research was supported in part by US National Institutes of Health (NIH) grants R37CA070867, R01CA082729, R01CA124558, R01CA148667 and R01CA122364, as well as by Ingram Professorship and Research Reward funds from the Vanderbilt University School of Medicine. Participating studies (grant support) in the consortium are as follows: Shanghai Women's Health Study (US NIH, R37CA070867), Shanghai Men's Health Study (US NIH, R01CA082729), Shanghai Breast and Endometrial Cancer Studies (US NIH, R01CA064277 and R01CA092585; contributing only controls), Guangzhou Colorectal Cancer Study (National Key Scientific and Technological Project, 2011ZX09307-001-04, and the National Basic Research Program, 2011CB504303, contributing only controls; the Natural Science Foundation of China, 81072383, contributing only controls), Aichi Colorectal Cancer Study (Grant-in-Aid for Cancer Research, the Grant for the Third Term Comprehensive Control Research for Cancer and Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, 17015018 and 221S0001), Korea–National Cancer Center Colorectal Cancer Study (Basic Science Research Program through the National Research Foundation of Korea, 2010-0010276; National Cancer Center Korea, 0910220), Korea-Seoul Colorectal Cancer Study (none reported) and KCPS-II colorectal Cancer Study (National R&D Program for Cancer Control, 0920330; Seoul R&D Program, 10526).
We wish to thank all participants, staff and investigators of GECCO and CCFR for making it possible to present the results in individuals of European ancestry for new CRC susceptibility loci identified in east Asians. Investigators (institution and location) from GECCO and CCFR who provided support for this project include (in alphabetical order) Aaron K. Aragaki (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), John A. Baron (Division of Gastroenterology and Hepatology, University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA), Sonja I. Berndt (Division of Cancer Epidemiology and Genetics, National Cancer Institute, US NIH, Bethesda, Maryland, USA), Stéphane Bezieau (Service de Génétique Médicale, Centre Hospitalier Universitaire (CHU) Nantes, Nantes, France), Hermann Brenner, Katja Butterbach (Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany), Bette J. Caan (Division of Research, Kaiser Permanente Medical Care Program, Oakland, California, USA), Christopher S. Carlson (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, and School of Public Health, University of Washington, Seattle, Washington, USA), Graham Casey (Department of Preventive Medicine, University of Southern California, Los Angeles, California, USA), Andrew T. Chan (Division of Gastroenterology, Harvard Medical School and Massachusetts General Hospital, Boston, Massachusetts, USA, and Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA), Jenny Chang-Claude (Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany), Stephen J. Chanock (Division of Cancer Epidemiology and Genetics, National Cancer Institute, US NIH, Bethesda, Maryland, USA), Lin S. Chen (Department of Health Studies, University of Chicago, Chicago, Illinois, USA), Gerhard A. Coetzee (Keck School of Medicine, University of Southern California, Los Angeles, California, USA), Simon G. Coetzee (Keck School of Medicine, University of Southern California, Los Angeles, California, USA), David V. Conti (Department of Preventive Medicine, University of Southern California, Los Angeles, California, USA), Keith Curtis (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), David Duggan (Translational Genomics Research Institute, Phoenix, Arizona, USA), Todd L. Edwards (Division of Epidemiology, Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee, USA), Charles S. Fuchs (Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA, and Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA), Steven Gallinger (Department of Surgery, Mount Sinai Hospital, Toronto, Ontario, Canada, and Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada), Edward L. Giovannucci (Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA, and Departments of Epidemiology and Nutrition, Harvard School of Public Health, Boston, Massachusetts, USA), Stephanie M. Gogarten (School of Public Health, University of Washington, Seattle, Washington, USA), Stephen B. Gruber (Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, California, USA), Robert W. Haile (Department of Preventive Medicine, University of Southern California, Los Angeles, California, USA), Tabitha A. Harrison (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), Richard B. Hayes (Division of Epidemiology, Department of Environmental Medicine, New York University School of Medicine, New York, New York, USA), Michael Hoffmeister (Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany), John L. Hopper (Melbourne School of Population Health, The University of Melbourne, Melbourne, Victoria, Australia), Li Hsu (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, and Department of Biostatistics, University of Washington, Seattle, Washington, USA), Thomas J. Hudson (Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada, and Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada), David J. Hunter (Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts, USA), Carolyn M. Hutter (Division of Cancer Control and Population Sciences, National Cancer Institute, US NIH, Bethesda, Maryland, USA), Rebecca D. Jackson (Division of Endocrinology, Diabetes, and Metabolism, Ohio State University, Columbus, Ohio, USA), Mark A. Jenkins (Melbourne School of Population Health, The University of Melbourne, Melbourne, Victoria, Australia), Shuo Jiao (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), Charles Kooperberg (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), Sébastien Küry (Service de Génétique Médicale, CHU Nantes, Nantes, France), Andrea Z. LaCroix (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), Cathy C. Laurie (Department of Biostatistics, University of Washington, Seattle, Washington, USA), Cecelia A. Laurie (Department of Biostatistics, University of Washington, Seattle, Washington, USA), Loic Le Marchand (Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu, Hawaii, USA), Mathieu Lemire (Ontario Institute for Cancer Research, Toronto, Ontario, Canada), David Levine (School of Public Health, University of Washington, Seattle, Washington, USA), Noralane M. Lindor (Department of Health Sciences Research, Mayo Clinic, Scottsdale, Arizona, USA), Yan Liu (Stephens and Associates, Carrollton, Texas, USA), Jing Ma (Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA), Karen W. Makar (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), Polly A. Newcomb (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, and Department of Epidemiology, University of Washington School of Public Health, Seattle, Washington, USA), Ulrike Peters (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, and Department of Epidemiology, University of Washington School of Public Health, Seattle, Washington, USA), John D. Potter (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, Department of Epidemiology, University of Washington School of Public Health, Seattle, Washington, USA, and Centre for Public Health Research, Massey University, Palmerston North, New Zealand), Ross L. Prentice (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), Conghui Qu (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA), Thomas Rohan (Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Yeshiva University, Bronx, New York, USA), Robert E. Schoen (Department of Medicine and Epidemiology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA), Fredrick R. Schumacher (Department of Preventive Medicine, University of Southern California, Los Angeles, California, USA), Daniela Seminara (Division of Cancer Control and Population Sciences, National Cancer Institute, US NIH, Bethesda, Maryland, USA), Martha L. Slattery (Department of Internal Medicine, University of Utah Health Sciences Center, Salt Lake City, Utah, USA), Darin Taverna (Translational Genomics Research Institute, Phoenix, Arizona, USA), Stephen N. Thibodeau (Department of Laboratory Medicine, Mayo Clinic, Rochester, Minnesota, USA, and Department of Pathology and Laboratory Genetics, Mayo Clinic, Rochester, Minnesota, USA), Cornelia M. Ulrich (Division of Preventive Oncology, German Cancer Research Center, Heidelberg, Germany, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, and Department of Epidemiology, University of Washington School of Public Health, Seattle, Washington, USA), Raakhee Vijayaraghavan (Genetic Basis of Human Disease Division, Translational Genomics Research Institute, Phoenix, Arizona, USA), Bruce Weir (Department of Biostatistics, University of Washington, Seattle, Washington, USA), Emily White (Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, and Department of Epidemiology, University of Washington School of Public Health, Seattle, Washington, USA) and Brent W. Zanke (Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Ontario, Canada).
We also thank B. Buecher of ASTERISK; U. Handte-Daub, M. Celik, R. Hettler-Jensen, U. Benscheid and U. Eilber of DACHS; P. Soule, H. Ranu, I. Devivo, D. Hunter, Q. Guo, L. Zhu and H. Zhang of HPFS, NHS and PHS; C. Berg and P. Prorok of PLCO; T. Riley of Information Management Services Inc.; B. O'Brien of Westat Inc; B. Kopp and W. Shao of SAIC-Frederick; investigators from the Women's Health Initiative (WHI; see URLs) and the GECCO Coordinating Center. Participating studies (grant support) in the GECCO and CCFR GWAS meta-analysis are as follows: GECCO (US NIH, U01 CA137088 and R01 CA059045), DALS (US NIH, R01 CA048998), Colo2&3 (US NIH, R01 CA060987), DACHS (German Federal Ministry of Education and Research, BR 1704/6-1, BR 1704/6-3, BR 1704/6-4, CH 117/1-1, 01KH0404 and 01ER0814), HPFS (US NIH, P01 CA055075, UM1 CA167552, R01 137178 and P50 CA127003), MEC (US NIH, R37 CA054281, P01 CA033619 and R01 CA063464), NHS (US NIH, R01 137178, P50 CA127003 and P01 CA087969), OFCCR (US NIH, U01 CA074783), PMH (US NIH, R01 CA076366), PHS (US NIH, CA042182), VITAL (US NIH, K05 CA154337), WHI (US NIH, HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, HHSN271201100004C and 268200764316C) and PLCO (US NIH, Z01 CP 010200, U01 HG004446 and U01 HG 004438). CCFR is supported by the National Cancer Institute, US NIH, under RFA CA-95-011 and through cooperative agreements with members of the Colon Cancer Family Registry and principal investigators of the Australasian Colorectal Cancer Family Registry (U01 CA097735), the Familial Colorectal Neoplasia Collaborative Group (U01 CA074799; USC), the Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01 CA074800), the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783), the Seattle Colorectal Cancer Family Registry (U01 CA074794) and the University of Hawaii Colorectal Cancer Family Registry (U01 CA074806). The GWAS work was supported by a National Cancer Institute grant (U01CA122839). OFCCR was supported by a GL2 grant from the Ontario Research Fund, the Canadian Institutes of Health Research and the Cancer Risk Evaluation (CaRE) Program grant from the Canadian Cancer Society Research Institute. B.Z. is a recipient of Senior Investigator Awards from the Ontario Institute for Cancer Research, through support from the Ontario Ministry of Economic Development and Innovation. ASTERISK was funded by a Regional Hospital Clinical Research Program (PHRC) and supported by the Regional Council of Pays de la Loire, Groupement des Entreprises Françaises dans la Lutte contre le Cancer (GEFLUC), Association Anne de Bretagne Génétique and Ligue Régionale Contre le Cancer (LRCC). PLCO data sets were accessed with approval through dbGaP (Cancer Genetic Markers of Susceptibility (CGEMS) prostate cancer scan, phs000207.v1.p1 and GWAS of Lung Cancer and Smoking, phs000093.v2.p2).
Author information
Authors and Affiliations
Consortia
Contributions
W.Z. conceived and directed ACCC as well as the Shanghai-Vanderbilt Colorectal Cancer Genetics Project. W.-H.J., Y.-X.Z., K.M., A.S., Y.-B.X., S.H.J., D.-H.K., U.P. and G.C. directed CRC projects at Guangzhou, Aichi, Korea-NCC, Shanghai, KCPS-II, Korea-Seoul, GECCO and CCFR, respectively. B.Z., Q.C. and W.W. coordinated the project. Q.C. directed laboratory operations. J.S. performed genotyping experiments. B.Z., J.L. and W.W. performed statistical analyses. W.Z. wrote the manuscript with substantial contributions from B.Z., Q.C., J.L., X.-O.S. and R.J.D. Z.R., G.Y., B.-T.J., Z.-Z.P., F.M., Y.-T.G., J.H.O., Y.-O.A., E.J.P., H.-L.L., J.W.P., J.J., J.-Y.J. and S.H. contributed to data and biological sample collection in the original studies included in ACCC and contributed to manuscript revision. Members of GECCO and CCFR contributed to data and biological sample collection in studies included in these consortia.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A complete list of members is provided in the Acknowledgements.
A complete list of members is provided in the Acknowledgements.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Tables 1–7 and Supplementary Figures 1–4 (PDF 2259 kb)
Rights and permissions
About this article
Cite this article
Jia, WH., Zhang, B., Matsuo, K. et al. Genome-wide association analyses in east Asians identify new susceptibility loci for colorectal cancer. Nat Genet 45, 191–196 (2013). https://doi.org/10.1038/ng.2505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2505
This article is cited by
-
Prioritization of risk genes in colorectal cancer by integrative analysis of multi-omics data and gene networks
Science China Life Sciences (2024)
-
Genetic risk impacts the association of menopausal hormone therapy with colorectal cancer risk
British Journal of Cancer (2024)
-
Well-TEMP-seq as a microwell-based strategy for massively parallel profiling of single-cell temporal RNA dynamics
Nature Communications (2023)
-
HSPA12A was identified as a key driver in colorectal cancer GWAS loci 10q26.12 and modulated by an enhancer–promoter interaction
Archives of Toxicology (2023)
-
Identification and functional validation of HLA-C as a potential gene involved in colorectal cancer in the Korean population
BMC Genomics (2022)