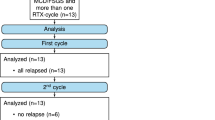
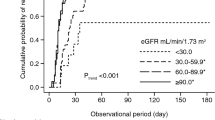
Abstract
Membranous nephropathy, focal segmental glomerulosclerosis and IgA nephropathy are the most commonly recognized types of primary glomerulonephritis that progress to end-stage renal disease. Persistent proteinuria is a major determinant of such progression. Reduction of proteinuria slows progression of renal disease and improves renal survival, but many of the agents used to reduce proteinuria carry a considerable risk of toxicity. The assessment of benefit versus risk of these medications can be further complicated by the temporal disconnect between the onset of benefit and of serious adverse events. In addition, relapses are common in these disorders and there is often a need for retreatment. Such retreatment might lead to repeated and/or prolonged drug exposure and to the oversight or underestimation of the cumulative dose of these agents because of the potentially extended interval between relapses. Consequently, it is very important to constantly review each patient's status and take into account their age, comorbid conditions and cumulative drug exposure when assessing treatment options. The potentially delayed development of adverse events also emphasizes the need for long-term surveillance of patients who receive immunosuppressive treatment for glomerular disease, often well beyond their drug exposure period and even when the treatment has been successful.
Key Points
-
In patients with membranous nephropathy and nephrotic-range proteinuria, achieving partial or complete remission of proteinuria slows renal function decline and improves long-term renal survival; both alkylating-agent-based and calcineurin-inhibitor-based regimens improve remission rates compared with placebo, but relapses are common and repeat treatment increases the total cumulative drug exposure
-
Nephrotoxicity is the major concern with calcineurin inhibitors, but their safety profile can be improved even during long-term exposure by use of monotherapy, close monitoring of drug level and renal function, and reduced target blood levels
-
Prognosis is poor in untreated patients with focal segmental glomerulosclerosis and nephrotic-range proteinuria; achieving even partial remission doubles the rate of renal survival at 10 years
-
The average age at presentation of focal segmental glomerulosclerosis has increased significantly over the past three decades, which has resulted in a significantly increased incidence of corticosteroid toxicity; ciclosporin is a suitable alternative to corticosteroids and induces at least partial remission in 60–70% of cases
-
For IgA nephropathy, blood-pressure control and renin–angiotensin system blockade remain the mainstay of treatment; corticosteroids and/or cytotoxic drugs are currently reserved for patients with sustained proteinuria and/or declining renal function
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ponticelli C et al. (1984) Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 310: 946–950
Troyanov S et al. (2004) Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int 66: 1199–1205
Laluck BJ Jr and Cattran DC (1999) Prognosis after a complete remission in adult patients with idiopathic membranous nephropathy. Am J Kidney Dis 33: 1026–1032
Ponticelli C et al. (1992) Remissions and relapses in idiopathic membranous nephropathy. Nephrol Dial Transplant 7 (Suppl 1): 85–90
Troyanov S et al. (2006) Renal pathology in idiopathic membranous nephropathy: a new perspective. Kidney Int 69: 1641–1648
Noel LH et al. (1979) Long-term prognosis of idiopathic membranous glomerulonephritis: study of 116 untreated patients. Am J Med 66: 82–90
Zucchelli P et al. (1987) Long-term outcome of idiopathic membranous nephropathy with nephrotic syndrome. Nephrol Dial Transplant 2: 73–78
MacTier R et al. (1986) The natural history of membranous nephropathy in the West of Scotland. Q J Med 60: 793–802
Marx BE and Marx M (1999) Prediction in idiopathic membranous nephropathy. Kidney Int 56: 666–673
Keith DS et al. (2004) Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663
Eriksen BO and Ingebretsen OC (2006) The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 69: 375–382
Ponticelli C et al. (1998) A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450
Jha V et al. (2007) A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904
Houssiau FA (2005) Cyclophosphamide in lupus nephritis. Lupus 14: 53–58
Faurschou M et al. (2008) Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol 35: 100–105
Branten AJ and Wetzels JF (2001) Short- and long-term efficacy of oral cyclophosphamide and steroids in patients with membranous nephropathy and renal insufficiency: study group. Clin Nephrol 56: 1–9
du Buf-Vereijken PW and Wetzels JF (2004) Efficacy of a second course of immunosuppressive therapy in patients with membranous nephropathy and persistent or relapsing disease activity. Nephrol Dial Transplant 19: 2036–2043
Reinhold-Keller E et al. (2000) An interdisciplinary approach to the care of patients with Wegener's granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 43: 1021–1032
Westman KW et al. (1998) Relapse rate, renal survival, and cancer morbidity in patients with Wegener's granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol 9: 842–852
Boumpas DT et al. (1993) Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med 119: 366–369
Mok CC et al. (1998) Risk factors for ovarian failure in patients with systemic lupus erythematosus receiving cyclophosphamide therapy. Arthritis Rheum 41: 831–837
Huong DL et al. (2002) Risk of ovarian failure and fertility after intravenous cyclophosphamide: a study in 84 patients. J Rheumatol 29: 2571–2576
Ioannidis JP et al. (2002) Predictors of sustained amenorrhea from pulsed intravenous cyclophosphamide in premenopausal women with systemic lupus erythematosus. J Rheumatol 29: 2129–2135
Mok CC et al. (2006) Long-term outcome of diffuse proliferative lupus glomerulonephritis treated with cyclophosphamide. Am J Med 119: 355.e25–355.e33
Wetzels JF (2004) Cyclophosphamide-induced gonadal toxicity: a treatment dilemma in patients with lupus nephritis? Neth J Med 62: 347–352
Meistrich ML et al. (1992) Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer 70: 2703–2712
Rivkees SA and Crawford JD (1988) The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA 259: 2123–2125
Moore RA and Derry S (2006) Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis. Arthritis Res Ther 8: R182
Ponticelli C et al. (1995) A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604
Branten AJ et al. (1998) Oral cyclophosphamide versus chlorambucil in the treatment of patients with membranous nephropathy and renal insufficiency. QJM 91: 359–366
The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group (1996) A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 61: 1029–1037
Ginzler EM et al. (2005) Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 353: 2219–2228
Robson R et al. (2005) Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 5: 2954–2960
Vial T and Descotes J (2003) Immunosuppressive drugs and cancer. Toxicology 185: 229–240
Andres A (2005) Cancer incidence after immunosuppressive treatment following kidney transplantation. Crit Rev Oncol Hematol 56: 71–85
Branten AJ et al. (2007) Mycophenolate mofetil in idiopathic membranous nephropathy: a clinical trial with comparison to a historic control group treated with cyclophosphamide. Am J Kidney Dis 50: 248–256
Cattran DC et al. (1995) A controlled trial of cyclosporine in patients with progressive membranous nephropathy: Canadian Glomerulonephritis Study Group. Kidney Int 47: 1130–1135
Cattran DC et al. (2001) Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int 59: 1484–1490
Praga M et al. (2007) Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int 71: 924–930
Lowe NJ et al. (1996) Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol 35: 710–719
Alexopoulos E et al. (2006) Induction and long-term treatment with cyclosporine in membranous nephropathy with the nephrotic syndrome. Nephrol Dial Transplant 21: 3127–3132
Kandaswamy R et al. (2007) Stable kidney function in the second decade after kidney transplantation while on cyclosporine-based immunosuppression. Transplantation 83: 722–726
Kasiske BL et al. (2003) Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185
van den Borne BE et al. (1998) No increased risk of malignancies and mortality in cyclosporin A-treated patients with rheumatoid arthritis. Arthritis Rheum 41: 1930–1937
Paul CF et al. (2003) Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol 120: 211–216
Sheashaa H et al. (2007) Does cyclosporine achieve a real advantage for treatment of idiopathic nephrotic syndrome in children? A long-term efficacy and safety study. Int Urol Nephrol 39: 923–928
Ruggenenti P et al. (2003) Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol 14: 1851–1857
Fervenza FC et al. (2008) Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117–125
Ruggenenti P et al. (2006) Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol 1: 738–748
Troyanov S et al. (2005) Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068
Cattran DC and Rao P (1998) Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis 32: 72–79
Rydel JJ et al. (1995) Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis 25: 534–542
Ponticelli C et al. (1999) Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? Am J Kidney Dis 34: 618–625
Kitiyakara C et al. (2004) Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825
Meyrier A et al. (1991) Treatment of adult idiopathic nephrotic syndrome with cyclosporin A: minimal-change disease and focal-segmental glomerulosclerosis. Collaborative Group of the French Society of Nephrology. Clin Nephrol 35 (Suppl 1): S37–S42
Ponticelli C et al. (1993) A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 43: 1377–1384
Lieberman KV and Tejani A (1996) A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 7: 56–63
Cattran DC et al. (1999) A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis: North America Nephrotic Syndrome Study Group. Kidney Int 56: 2220–2226
Cattran DC et al. (2007) Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: workshop recommendations. Kidney Int 72: 1429–1447
Tumlin JA et al. (2006) A prospective, open-label trial of sirolimus in the treatment of focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 1: 109–116
Cho ME et al. (2007) Sirolimus therapy of focal segmental glomerulosclerosis is associated with nephrotoxicity. Am J Kidney Dis 49: 310–317
Simon P et al. (2004) Epidemiologic data of primary glomerular diseases in western France. Kidney Int 66: 905–908
Li LS and Liu ZH (2004) Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923
Alamartine E et al. (1991) Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis 18: 12–19
Rekola S et al. (1991) Deterioration of GFR in IgA nephropathy as measured by 51Cr-EDTA clearance. Kidney Int 40: 1050–1054
Reich HN et al. (2007) Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183
Bartosik LP et al. (2001) Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735
Kanno Y et al. (2000) Blood pressure reduction associated with preservation of renal function in hypertensive patients with IgA nephropathy: a 3-year follow-up. Clin Nephrol 54: 360–365
Nakao N et al. (2003) Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet 361: 117–124
Praga M et al. (2003) Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol 14: 1578–1583
Coppo R et al. (2007) IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol 18: 1880–1888
Li PK et al. (2006) Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis 47: 751–760
Palmer BF (2004) Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 351: 585–592
Vleeming W et al. (1998) ACE inhibitor-induced angioedema: incidence, prevention and management. Drug Saf 18: 171–188
Wood R (1995) Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril: a controlled retrospective cohort study. Br J Clin Pharmacol 39: 265–270
Shotan A et al. (1994) Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med 96: 451–456
Cooper WO et al. (2006) Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 354: 2443–2451
Donadio JV Jr et al. (1994) A controlled trial of fish oil in IgA nephropathy: Mayo Nephrology Collaborative Group. N Engl J Med 331: 1194–1199
Dillon JJ (1997) Fish oil therapy for IgA nephropathy: efficacy and interstudy variability. J Am Soc Nephrol 8: 1739–1744
Akagi H et al. (2004) Long-term results of tonsillectomy as a treatment for IgA nephropathy. Acta Otolaryngol Suppl 555: 38–42
Xie Y et al. (2004) Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int 65: 1135–1144
Wei JL et al. (2000) Evaluation of posttonsillectomy hemorrhage and risk factors. Otolaryngol Head Neck Surg 123: 229–235
Pozzi C et al. (1999) Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 353: 883–887
Pozzi C et al. (2004) Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163
Goumenos DS et al. (2003) Prednisolone and azathioprine in IgA nephropathy—a ten-year follow-up study. Nephron Clin Pract 93: c58–c68
Tang S et al. (2005) Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 68: 802–812
Maes BD et al. (2004) Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int 65: 1842–1849
Frisch G et al. (2005) Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dial Transplant 20: 2139–2145
Ballardie FW and Roberts IS (2002) Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 13: 142–148
Acknowledgements
D Philibert is supported in part by La Faculté de Médecine de l'Université Laval à Québec.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Philibert, D., Cattran, D. Remission of proteinuria in primary glomerulonephritis: we know the goal but do we know the price?. Nat Rev Nephrol 4, 550–559 (2008). https://doi.org/10.1038/ncpneph0915
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0915
This article is cited by
-
Adrenocorticotropic hormone therapy for the treatment of idiopathic nephrotic syndrome in children and young adults: a systematic review of early clinical studies with contemporary relevance
Journal of Nephrology (2017)
-
Acthar gel in the treatment of nephrotic syndrome: a multicenter retrospective case series
BMC Nephrology (2016)
-
Rituximab in adult patients with multi-relapsing/steroid-dependent minimal change disease and focal segmental glomerulosclerosis: a report of 5 cases
Wiener klinische Wochenschrift (2013)
-
Chapter 2: General principles in the management of glomerular disease
Kidney International Supplements (2012)
-
References
Kidney International Supplements (2012)