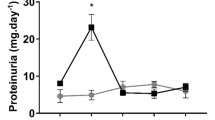
Abstract
The mechanisms that underlie tolerance to injury in immature animals and tissues have been a subject of interest since 1670. Observations in neonatal units that premature infants are less prone to develop acute renal failure than adults in critical care units have prompted a series of investigations. Although initially attributed to metabolic adaptation such as increased glycolytic capacity and preservation of high energy phosphate, more recent studies have indicated a prominent role for the heat shock response. Observed modulations of injury by heat shock proteins in the immature kidney have significant implications for advancement of our understanding of renal cell injury in both adults and children.
Key Points
-
The incidence of injury to the neonatal kidney, relative to the mature kidney, is lower than projected
-
In animal models, the heat shock response has a role in tolerance of the immature kidney to injury
-
Experimental inhibition of the heat shock response in immature renal tubules increases susceptibility to injury
-
'Priming' the heat shock response of the mature kidney, either pharmacologically or via a preconditioning insult, confers protection against a subsequent injurious challenge
-
Profiling patient expression of genes involved in the heat shock response might identify those at greatest risk of acute kidney injury
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gouyon JB and Guignard JP (2000) Management of acute renal failure in newborns. Pediatr Nephrol 14: 1037–1044
Haycock GB (2003) Management of acute and chronic renal failure in the newborn. Semin Neonatol 8: 325–334
Toth-Heyn P et al. (2000) The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol 14: 227–239
Andreoli SP (2004) Acute renal failure in the newborn. Semin Perinatol 28: 112–123
Drukker A and Guignard JP (2002) Renal aspects of the term and preterm infant: a selective update. Curr Opin Pediatr 14: 175–182
Tapia-Rombo CA et al. (1997) Usefulness of fractional excretion of sodium in critically ill pre-term newborns. Arch Med Res 28: 253–257
Mathew OP et al. (1980) Neonatal renal failure: usefulness of diagnostic indices. Pediatrics 65: 57–60
Mishra J et al. (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238
Vasarhelyi B et al. (2005) Genetic polymorphisms and risk for acute renal failure in preterm neonates. Pediatr Nephrol 20: 132–135
Schrier RW et al. (2004) Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114: 5–14
Bonventre JV and Weinberg JM (2003) Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210
Molitoris BA and Sutton TA (2004) Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int 66: 496–499
Boyle R (1670) New pneumatical experiments about respiration. Phil Trans 5: 2011–2031
Le Gallois JJC (1813) Experiments on the principle of life. Philadelphia: M Thomas
Constantinescu AR et al. (1996) Age dependence of tolerance to anoxia and changes in cytosolic calcium in rabbit renal proximal tubules. Pediatr Nephrol 10: 606–612
Fazekas JF et al. (1941) Tolerance of the newborn to anoxia. Am J Physiol 134: 281–287
Weismann DN and Clarke WR (1981) Postnatal age-related renal responses to hypoxemia in lambs. Circ Res 49: 1332–1338
Whittam R (1961) Metabolic changes in rabbit kidney cortex during the first few weeks after birth. Biochim Biophys Acta 54: 574–576
Swaab DF and Boer K (1972) The presence of biologically labile compounds during ischemia and their relationship to the EEG in rat cerebral cortex and hypothalamus. J Neurochem 19: 2843–2853
Fischer JH and Isselhard W (1975) Metabolic patterns in several tissues of newborn rabbits during ischemia. Biol Neonate 27: 235–250
Gaudio KM et al. (1996) Glycolysis is not responsible for the tolerance of immature renal tubules to anoxia. Pediatr Res 40: 457–461
Morimoto RI et al. (1992) Transcriptional regulation of heat shock genes: a paradigm for inducible genomic responses. J Biol Chem 267: 21987–21990
Van Why SK et al. (1992) Induction and intracellular localization of HSP-72 after renal ischemia. Am J Physiol 263: F769–F775
Van Why SK et al. (1994) Activation of heat-shock transcription factor by graded reductions in renal ATP, in vivo, in the rat. J Clin Invest 94: 1518–1523
Aufricht C et al. (1998) Heat-shock protein 25 induction and redistribution during actin reorganization after renal ischemia. Am J Physiol 274: F215–F222
Emami A et al. (1991) Transient ischemia or heat stress induces a cytoprotectant protein in rat kidney. Am J Physiol 260: F479–F485
Park KM et al. (2005) Orchiectomy reduces susceptibility to renal ischemic injury: a role for heat shock proteins. Biochem Biophys Res Commun 328: 312–317
Gaudio KM et al. (1998) Role of heat stress response in the tolerance of immature renal tubules to anoxia. Am J Physiol 274: F1029–F1036
Vicencio A et al. (2003) Developmental expression of HSP-72 and ischemic tolerance of the immature kidney. Pediatr Nephrol 18: 85–91
Sreedharan R et al. (2005) Reduced tolerance of immature renal tubules to anoxia by HSF-1 decoy. Am J Physiol Renal Physiol 288: F322–F326
Riordan M et al. (2004) Differential inhibition of HSP72 and HSP25 produces profound impairment of cellular integrity. J Am Soc Nephrol 15: 1557–1566
Amoah-Apraku B et al. (2000) A non-nucleotide-bridged DNA decoy inhibits renal epithelial nitric oxide synthase expression. Kidney Int 57: 83–91
Gao H et al. (1994) Double-stranded cyclic oligonucleotides with non-nucleotide bridges. Bioconjug Chem 5: 445–453
Rowland RT et al. (1995) Mechanisms of immature myocardial tolerance to ischemia: phenotypic differences in antioxidants, stress proteins, and oxidases. Surgery 118: 446–452
Adachi S et al. (2004) Cellular response to renal hypoxia is different in adolescent and infant rats. Pediatr Res 55: 485–491
Park KM et al. (2001) Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276: 11870–11876
Borkan SC et al. (1993) Heat stress protein-associated cytoprotection of inner medullary collecting duct cells from rat kidney. Am J Physiol 265: F333–F341
Aufricht C et al. (2002) Ischemic conditioning prevents Na,K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res 51: 722–727
Rauchman MI et al. (1997) Induction of molecular chaperones by hyperosmotic stress in mouse inner medullary collecting duct cells. Am J Physiol 273: F9–F17
Nissim I et al. (1992) A mechanism of glycine and alanine cytoprotective action: stimulation of stress-induced HSP70 mRNA. Kidney Int 42: 775–782
Chopp M et al. (1989) Transient hyperthermia protects against subsequent forebrain ischemic cell damage in the rat. Neurology 39: 1396–1398
Currie RW et al. (1988) Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res 63: 543–549
Donnelly TJ et al. (1992) Heat shock protein induction in rat hearts: a role for improved myocardial salvage after ischemia and reperfusion? Circulation 85: 769–778
Yang CW et al. (2003) Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J 17: 1754–1755
Nietzsche F (1889) Die Götzen-Dämmerung (Twilight of the Idols).
Van Why SK et al. (2003) Hsp27 associates with actin and limits injury in energy depleted renal epithelia. J Am Soc Nephrol 14: 98–106
Molitoris BA et al. (1992) Cytoskeleton disruption and apical redistribution of proximal tubule Na(+)-K(+)-ATPase during ischemia. Am J Physiol 263: F488–F495
Molitoris BA et al. (1991) Dissociation and redistribution of Na+,K(+)-ATPase from its surface membrane actin cytoskeletal complex during cellular ATP depletion. J Clin Invest 88: 462–469
Riordan M et al. (2005) HSP70 binding modulates detachment of Na-K-ATPase following energy deprivation in renal epithelial cells. Am J Physiol Renal Physiol 288: F1236–F1242
Herget-Rosenthal S et al. (2001) Characteristics of EYFP-actin and visualization of actin dynamics during ATP depletion and repletion. Am J Physiol Cell Physiol 281: C1858–C1870
Shelden EA et al. (2002) Heat shock protein 27 associates with basolateral cell boundaries in heat-shocked and ATP-depleted epithelial cells. J Am Soc Nephrol 13: 332–341
Kelly KJ et al. (2001) Induction of stress response proteins and experimental renal ischemia/reperfusion. Kidney Int 59: 1798–1802
Trinklein ND et al. (2004) The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell 15: 1254–1261
Elbashir SM et al. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498
Fekete A et al. (2003) Association between heat shock protein 72 gene polymorphism and acute renal failure in premature neonates. Pediatr Res 54: 452–455
Vasarhelyi B et al. (2005) Genetic polymorphisms and risk for acute renal failure in preterm neonates. Pediatr Nephrol 20: 132–135
Acknowledgements
We would like to thank the large number of colleagues and collaborators who have contributed to the studies from our laboratory that are reviewed here. We appreciate funding from the NIH (PO1-HD-32573 and PO1-DK-17433), National Kidney Foundation, American Heart Association and the Eden Fellowship (Royal College of Physicians, London).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Riordan, M., Sreedharan, R., Kashgarian, M. et al. Modulation of renal cell injury by heat shock proteins: lessons learned from the immature kidney. Nat Rev Nephrol 2, 149–156 (2006). https://doi.org/10.1038/ncpneph0117
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0117