Abstract
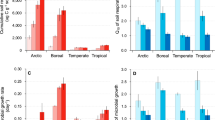
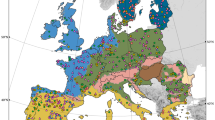
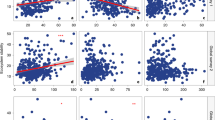
Soil fungi have pivotal ecological roles as decomposers, pathogens and symbionts1,2. Alterations to their diversity arising from climate change could have substantial effects on ecosystems, particularly those undergoing rapid warming that contain few species3,4. Here, we report a study using pyrosequencing to assess fungal diversity in 29 soils sampled from a 1,650 km climatic gradient through the maritime Antarctic, the most rapidly warming region in the Southern Hemisphere5,6. Using a ‘space-for-time’ substitution approach, we show that soil fungal diversity is higher in warmer habitats, with increases of 4.7 (observed) and 11.3 (predicted) fungal taxa per degree Celsius rise in surface temperature along the transect. Among 22 predictor variables, air temperature was the strongest and most consistent predictor of diversity. We propose that the current rapid warming in the maritime Antarctic (0.34 °C per decade6) will facilitate the colonization of soil by a wider diversity of fungi than at present, with data from regression models suggesting 20–27% increases in fungal species richness in the southernmost soils by 2100. Such increases in diversity, which provide a sentinel for changes at lower latitudes, are likely to have substantial effects on nutrient cycling and, ultimately, productivity in the species-poor soils of maritime Antarctica.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Swift, M. J., Heal, O. W. & Anderson, J. M. Decomposition in Terrestrial Ecosystems (Blackwell Scientific Publications, 1979).
Tedersoo, L. et al. Global diversity and geography of soil fungi. Science 346, 1078 (2014).
Setälä, H. & McLean, M. A. Decomposition rate of organic substrates in relation to the species diversity of soil saprophytic fungi. Oecologia 139, 98–107 (2004).
Tiunov, A. & Scheu, S. Facilitative interactions rather than resource partitioning drive diversity-functioning relationships in laboratory fungal communities. Ecol. Lett. 8, 618–625 (2005).
Adams, B. et al. in Antarctic Climate Change and the Environment (eds Turner, J. et al.) 183–298 (Scientific Committee on Antarctic Research, Scott Polar Research Institute, 2009).
Turner, J. et al. Antarctic climate change and the environment: An update. Polar Rec. 50, 237–259 (2014).
Convey, P. & Smith, R. I. L. Responses of terrestrial Antarctic ecosystems to climate change. Plant Ecol. 182, 1–10 (2006).
Fowbert, J. A. & Smith, R. I. L. Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arctic Alpine Res. 26, 290–296 (1994).
Frenot, Y. et al. Biological invasions in the Antarctic: Extent, impacts and implications. Biol. Rev. 80, 45–72 (2005).
Royles, J. et al. Plants and soil microbes respond to recent warming on the Antarctic Peninsula. Curr. Biol. 23, 1702–1706 (2013).
Hill, P. W. et al. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nature Clim. Change 1, 50–53 (2011).
Yergeau, E. et al. Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J. 6, 692–702 (2011).
Dennis, P. G. et al. Warming constrains bacterial community responses to nutrient inputs in a southern, but not northern, maritime Antarctic soil. Soil Biol. Biochem. 57, 248–255 (2013).
Green, T. G., Brabyn, L., Beard, C. & Sancho, L. G. Extremely low lichen growth rates in Taylor Valley, Dry Valleys, continental Antarctica. Polar Biol. 35, 535–541 (2012).
Deslippe, J. R., Hartmann, M., Mohn, W. W. & Simard, S. W. Long-term experimental manipulation of climate alters the ectomycorrhizal community of Betula nana in Arctic tundra. Glob. Change Biol. 17, 1625–1636 (2011).
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural ecosystems. Nature 421, 37–42 (2003).
Bragazza, L., Parisod, J., Buttler, A. & Bardgett, R. D. Biogeochemical plant-soil microbe feedback in response to climate warming in peatlands. Nature Clim. Change 3, 273–277 (2013).
VanLipzig, N. P. M., Van Meijgaard, E. & Oerlemans, J. Evaluation of a regional atmospheric model using measurements of surface heat exchange processes from a site in Antarctica. Mon. Weath. Rev. 127, 1994–2011 (1999).
Callesen, I., Rauland-Rasmussen, K., Westman, C. J. & Tau-Strand, L. Nitrogen pools and C:N ratios in well-drained Nordic forest soils related to climate and soil texture. Boreal Environ. Res. 12, 681–692 (2007).
Osono, T., Matsuoka, S., Hirose, D., Uchida, M. & Kanda, H. Fungal colonization and decomposition of leaves and stems of Salix arctica on deglaciated moraines in high-Arctic Canada. Polar Sci. 8, 207–216 (2014).
Øvstedal, D. O. & Smith, R. I. L. Lichens of Antarctica and South Georgia: A Guide to their Identification and Ecology (Studies in Polar Research, Cambridge Univ. Press, 2001).
Peat, H. J., Clarke, A. & Convey, P. Diversity and biogeography of the Antarctic flora. J. Biogeogr. 34, 132–146 (2007).
Pointing, S. B. et al. Highly specialized microbial diversity in hyper-arid polar desert. Proc. Natl Acad. Sci. USA 106, 19964–19969 (2009).
Green, T. G., Sancho, L. G., Pintado, A. & Schoeter, B. Functional and spatial pressures on terrestrial vegetation in Antarctica forced by global warming. Polar Biol. 34, 1643–1656 (2011).
Upson, R., Newsham, K. K. & Read, D. J. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 20, 1–11 (2009).
Chan, Y., Van Nostrand, J. D., Zhou, J., Pointing, S. B. & Farrell, R. L. Functional ecology of an Antarctic Dry Valley. Proc. Natl Acad. Sci. USA 110, 8990–8995 (2013).
Ihrmark, K. et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 82, 666–677 (2012).
White, T. J., Bruns, T. D., Lee, S. B. & Taylor, J. W. in PCR Protocols: A Guide to Methods and Applications (eds Innis, M. A., Gelfand, H., Sninsky, J. S. & White, T. J.) 315–321 (Academic Press, 1990).
Margulies, M. et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437, 376–380 (2005).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7, 335–336 (2010).
Bragg, L., Stone, G., Imelfort, M., Hugenholtz, P. & Tyson, G. W. Fast, accurate error-correction of amplicon pyrosequences using Acacia. Nature Methods 9, 425–426 (2012).
Bengtsson-Palme, J. et al. ITSx: Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for use in environmental sequencing. Methods Ecol. Evol. 4, 914–919 (2013).
Nilsson, R. H. et al. A comprehensive, automatically updated fungal ITS sequence dataset for reference-based chimera control in environmental sequencing efforts. Microbes Environ. 30, 145–150 (2015).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Kõljalg, U. et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 22, 5271–5277 (2013).
Acknowledgements
Logistical support was provided by the British Antarctic Survey and the Royal Navy (HMS Endurance). V. A. Laudicina, V. Ord, P. Coates, M. Dunn, P. Torode, M. Jobson, A. Clark, J. Wake, D. Hall, G. Marshall, M. Biszczuk and K. Bazeley provided technical support. This work was funded by a UK Natural Environment Research Council Antarctic Funding Initiative grant (NE/D00893X/1; AFI 7/05) led by D.W.H. and a University of Queensland Early Career Researcher Award to P.G.D. All are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
K.K.N., P.G.D., S.P.R., A.G.O’D. and D.W.H. conceived this study. P.G.D., K.K.N. and D.W.H. collected soil samples, L.C.C. performed DNA extractions and PCRs. P.G.D. processed sequence data and P.G.D. and K.K.N. performed statistical analyses. P.T.F. provided geospatial data derived from the Regional Atmospheric Climate Model. All authors discussed the results and contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Newsham, K., Hopkins, D., Carvalhais, L. et al. Relationship between soil fungal diversity and temperature in the maritime Antarctic. Nature Clim Change 6, 182–186 (2016). https://doi.org/10.1038/nclimate2806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate2806
This article is cited by
-
Rapid growth rate responses of terrestrial bacteria to field warming on the Antarctic Peninsula
The ISME Journal (2023)
-
Plant and fungal species interactions differ between aboveground and belowground habitats in mountain forests of eastern China
Science China Life Sciences (2023)
-
Archaeal and Bacterial Diversity and Distribution Patterns in Mediterranean-Climate Vernal Pools of Mexico and the Western USA
Microbial Ecology (2023)
-
A comprehensive assessment of fungal communities in various habitats from an ice-free area of maritime Antarctica: diversity, distribution, and ecological trait
Environmental Microbiome (2022)
-
Metabarcoding analysis of the soil fungal community to aid the conservation of underexplored church forests in Ethiopia
Scientific Reports (2022)