Abstract
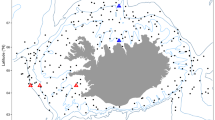
Arctic marine ecosystems are warming twice as fast as the global average1. As a consequence of warming, many incoming species experience increasing abundances and expanding distribution ranges in the Arctic2. The Arctic is expected to have the largest species turnover with regard to invading and locally extinct species, with a modelled invasion intensity of five times the global average3. Studies in this region might therefore give valuable insights into community-wide shifts of species driven by climate warming. We found that the recent warming in the Barents Sea4 has led to a change in spatial distribution of fish communities, with boreal communities expanding northwards at a pace reflecting the local climate velocities5. Increased abundance and distribution areas of large, migratory fish predators explain the observed community-wide distributional shifts. These shifts change the ecological interactions experienced by Arctic fish species. The Arctic shelf fish community retracted northwards to deeper areas bordering the deep polar basin. Depth might limit further retraction of some of the fish species in the Arctic shelf community. We conclude that climate warming is inducing structural change over large spatial scales at high latitudes, leading to a borealization of fish communities in the Arctic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hoegh-Guldberg, O. & Bruno, J. F. The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528 (2010).
Doney, S. C. et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37 (2012).
Cheung, W. W. L. et al. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 10, 235–251 (2009).
Smedsrud, L. H. et al. The role of the Barents Sea in the Arctic climate system. Rev. Geophys. 51, 415–449 (2013).
Pinsky, M. L., Worm, B., Fogarty, M. J., Sarmiento, J. L. & Levin, S. A. Marine taxa track local climate velocities. Science 341, 1239–1242 (2013).
Sunday, J. M., Bates, A. E. & Dulvyy, N. K. Thermal tolerance and global redistribution of animals. Nature Clim. Change 2, 686–690 (2012).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nature Clim. Change 3, 919–925 (2013).
Richardson, A. J. et al. Climate change and marine life. Biol. Lett. 8, 907–909 (2012).
Cheung, W. W. L., Watson, R. & Pauly, D. Signature of ocean warming in global fisheries catch. Nature 497, 365–367 (2013).
Simpson, S. D. et al. Continental shelf-wide response of a fish assemblage to rapid warming of the sea. Curr. Biol. 21, 1565–1570 (2011).
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005).
Dulvy, N. K. et al. Climate change and deepening of the North Sea fish assemblage: A biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039 (2008).
Hollowed, A. B., Planque, B. & Loeng, H. Potential movement of fish and shellfish stocks from the sub-Arctic to the Arctic Ocean. Fish. Oceanogr. 22, 355–370 (2013).
Comiso, J. C. Large decadal decline of the Arctic multiyear ice cover. J. Clim. 25, 1176–1193 (2012).
Johannesen, E., Høines, Å. S., Dolgov, A. V. & Fossheim, M. Demersal fish assemblages and spatial diversity patterns in the Arctic–Atlantic transition zone in the Barents Sea. PLoS ONE 7, e34924 (2012).
Spielhagen, R. F. et al. Enhanced modern heat transfer to the Arctic by warm Atlantic water. Science 331, 450–453 (2011).
Wassmann, P. et al. Food webs and carbon flux in the Barents Sea. Prog. Oceanogr. 71, 232–287 (2006).
Kjesbu, O. S. et al. Synergies between climate and management for Atlantic cod fisheries at high latitudes. Proc. Natl Acad. Sci. USA 111, 3478–3483 (2014).
Renaud, P. E. et al. Is the poleward expansion by Atlantic cod and haddock threatening native polar cod, Boreogadus saida? Polar Biol. 35, 401–412 (2012).
Johansen, G. O., Johannesen, E., Michalsen, K., Aglen, A. & Fotland, Å. Seasonal variation in geographic distribution of North East Arctic (NEA) cod–survey coverage in a warmer Barents Sea. Mar. Biol. Res. 9, 908–919 (2013).
Landa, C. S., Ottersen, G., Sundby, S., Dingsør, G. E. & Stiansen, J. E. Recruitment, distribution boundary and habitat temperature of an arcto-boreal gadoid in a climatically changing environment: A case study on Northeast Arctic haddock (Melanogrammus aeglefinus). Fish. Oceanogr. 23, 506–520 (2014).
Dalpadado, P. et al. Productivity in the Barents Sea–Response to recent climate variability. PLoS ONE 9, e95273 (2014).
Genner, M. J. et al. Regional climatic warming drives long-term community changes of British marine fish. Proc. R. Soc. Lond. 271, 655–661 (2004).
Dalpadado, P. et al. Climate effects on Barents Sea ecosystem dynamics. ICES J. Mar. Sci. 69, 1303–1316 (2012).
Falk-Petersen, S., Mayzaud, P., Kattner, G. & Sargent, J. R. Lipids and life strategy of Arctic Calanus. Mar. Biol. Res. 5, 18–39 (2009).
Cochrane, S. K. J. et al. Benthic macrofauna and productivity regimes in the Barents Sea—Ecological implications in a changing Arctic. J. Sea Res. 61, 222–233 (2009).
Planque, B. et al. Who eats whom in the Barents Sea: A food web topology from plankton to whales. Ecol. Arch. 95, E095–E124 (2014).
Wiedmann, M. A., Primicerio, R., Dolgov, A., Ottesen, C. A. M. & Aschan, M. Life history variation in Barents Sea fish: Implications for sensitivity to fishing in a changing environment. Ecol. Evol. 4, 3596–3611 (2014).
Hop, H. & Gjøsæter, H. Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea. Mar. Biol. Res. 9, 878–894 (2013).
Wiedmann, M. A. et al. Functional diversity of the Barents Sea fish community. Mar. Ecol. Prog. Ser. 495, 205–218 (2014).
Cavalieri, D. J., Parkinson, C. L., Gloersen, P. & Zwally, H. J. Sea Ice Concentrations from Nimbus-7 SMMR and DMSP SSM/I Passive Microwave Data. Digital Media (National Snow and Ice Data Center, 1996); http://nsidc.org/data/nsidc-0051.html
Boitsov, V. D., Karsakov, A. L. & Trofimov, A. G. Atlantic water temperature and climate in the Barents Sea, 2000–2009. ICES J. Mar. Sci. 69, 833–840 (2012).
Cressie, N. A. C. Statistics for Spatial Data (Wiley, 1993).
Greenacre, M. & Primicerio, R. Multivariate Analysis of Ecological Data (Fundación BBVA, 2013).
Legendre, P. & Legendre, L. F. J. Numerical Ecology 3rd edn (Elsevier, 2012).
Elith, J. & Leathwick, J. R. Species distribution models. Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697 (2009).
Dormann, J. C. et al. Collinearity: A review of methods to deal with it and a simulation study to evaluate their performance. Ecography 36, 27–46 (2013).
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, S. M. Mixed Effects Models and Extensions in Ecology with R (Springer, 2009).
Acknowledgements
This is a contribution to the BarEcoRe (Barents Sea ecosystem resilience under global environmental change) project, funded by the Norwegian Research Council (200793/S30). We would like to thank everyone involved in the joint ecosystem surveys at the Institute of Marine Research (IMR), Norway, and Knipovich Polar Research Institute of Marine Fisheries and Oceanography (PINRO), Russia.
Author information
Authors and Affiliations
Contributions
M.F., R.P. and E.J. contributed to the formulation of hypotheses and approach. M.F., R.P. and R.B.I. performed the data analysis. All authors participated in writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Fossheim, M., Primicerio, R., Johannesen, E. et al. Recent warming leads to a rapid borealization of fish communities in the Arctic. Nature Clim Change 5, 673–677 (2015). https://doi.org/10.1038/nclimate2647
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate2647
This article is cited by
-
More frequent atmospheric rivers slow the seasonal recovery of Arctic sea ice
Nature Climate Change (2023)
-
Pan-Arctic marine biodiversity and species co-occurrence patterns under recent climate
Scientific Reports (2023)
-
Diets of gadoid fish in Arctic waters of Svalbard fjords during the polar night
Polar Biology (2023)
-
Temporal dynamics and environmental drivers of polar cod (Boreogadus saida) densities in the northeast Chukchi Sea
Polar Biology (2023)
-
Predicting impacts of climate change on the biogeographic patterns of representative species richness in Prydz Bay-Amery Ice Shelf
Journal of Oceanology and Limnology (2023)