Abstract
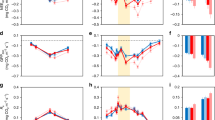
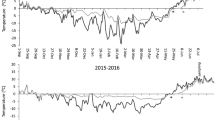
Climate change is affecting plant community composition1 and ecosystem structure, with consequences for ecosystem processes such as carbon storage2,3,4. Climate can affect plants directly by altering growth rates1, and indirectly by affecting predators and herbivores, which in turn influence plants5,6,7,8,9. Diseases are also known to be important for the structure and function of food webs10,11,12,13,14. However, the role of plant diseases in modulating ecosystem responses to a changing climate is poorly understood15,16. This is partly because disease outbreaks are relatively rare and spatially variable, such that that their effects can only be captured in long-term experiments. Here we show that, although plant growth was favoured by the insulating effects of increased snow cover in experimental plots in Sweden, plant biomass decreased over the seven-year study. The decline in biomass was caused by an outbreak of a host-specific parasitic fungus, Arwidssonia empetri, which killed the majority of the shoots of the dominant plant species, Empetrum hermaphroditum, after six years of increased snow cover. After the outbreak of the disease, instantaneous measurements of gross photosynthesis and net ecosystem carbon exchange were significantly reduced at midday during the growing season. Our results show that plant diseases can alter and even reverse the effects of a changing climate on tundra carbon balance by altering plant composition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Walker, M. D. et al. Plant community responses to experimental warming across the tundra biome. Proc. Natl Acad. Sci. USA 103, 1342–1346 (2006).
Chapin, F. S. III et al. Role of land-surface changes in arctic summer warming. Science 310, 657–660 (2005).
Mack, M. C. et al. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431, 440–443 (2004).
Dorrepaal, E. et al. 2009. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460, 616–620 (2009).
Post, E. & Pedersen, C. Opposing plant community responses to warming with and without herbivores. Proc. Natl Acad. Sci. USA 105, 12353–12357 (2008).
Engelkes, T. et al. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456, 946–948 (2008).
Olofsson, J. et al. Herbivores inhibit climate-driven shrub expansion on the tundra. Glob. Change Biol. 15, 2681–2693 (2009).
Post, E. et al. Ecological dynamics across the arctic associated with recent climate change. Science 325, 1355–1358 (2009).
Sjögersten, S., Van der Wal, R. & Woodin, S. J. Habitat type determines herbivory controls over CO2 fluxes in a warmer arctic. Ecology 89, 2103–2116 (2008).
Nordin, A., Strengbom, J., Forsum, A. & Ericson, L. Complex biotic interactions drive long-term vegetation change in a nitrogen enriched boreal forest. Ecosystems 12, 1204–1211 (2009).
Borer, E. T., Hosseini, P. R., Seabloom, E. W. & Dobson, A. P. Pathogen-induced reversal of native dominance in a grassland community. Proc. Natl Acad. Sci. USA 104, 5473–5478 (2009).
Holdo, R. M. et al. A disease-mediated trophic cascade in the Serengeti and its implications for ecosystem C. PLoS Biol. 7, e1000210 (2009).
Wilmers, C. C. et al. Predator disease out-break modulates top-down, bottom-up and climatic effects on herbivore population dynamics. Ecol. Lett. 9, 383–389 (2006).
Roy, B. A., Gusewell, S. & Harte, J. Response of plant pathogens and herbivores to a warming experiment. Ecology 85, 2570–2581 (2004).
Wiedermann, M. M. et al. Global change shifts vegetation and plant–parasite interactions in a boreal mire. Ecology 88, 454–464 (2007).
Harvell, C. D. et al. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2163 (2002).
Vestergren, T. Om den olikformiga snöbeteckningens inflytande på vegetationen i Sarekfjällen. Bot. Not 5, 241–268 (1902).
Wipf, S. & Rixen, C. A review of snow manipulation experiments in arctic and alpine ecosystems. Polar Res. 29, 95–109 (2010).
Schimel, J. P., Bilbrough, C. & Welker, J. M. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biol. Biochem. 36, 217–227 (2004).
Buckeridge, K. M. & Grogan, P. Deepened snow alters soil microbial nutrient limitations in arctic birch hummock tundra. Appl. Soil Ecol. 39, 210–222 (2008).
Bokhorst, S. F., Bjerke, J. W., Tømmervik, H., Callaghan, T. V. & Phoenix, G. K. Winter warming events damage sub-Arctic vegetation: Consistent evidence from an experimental manipulation and a natural event. J. Ecol. 97, 1408–1415 (2009).
Nilsson, M. C. & Wardle, D. A. Understory vegetation as a forest ecosystem driver: Evidence from northern Swedish boreal forest. Front. Ecol. Environ. 3, 421–428 (2005).
Strengbom, J. et al. Parasitic fungus mediates change in nitrogen-exposed boreal forest vegetation. J. Ecol. 90, 61–67 (2002).
Wahren, C. H. A, Walker, M. D. & Bret-Harte, M. S. Vegetation responses in Alaskan arctic tundra after 8 years of summer warming and winter snow manipulation experiment. Glob. Change Biol. 11, 537–552 (2005).
Welker, J. M., Fahnestock, J. T. & Jones, M. H. Annual CO2 flux in dry and moist arctic tundra: Field responses to increases in summer temperatures and winter snow depth. Clim. Change 44, 139–150 (2000).
Nobrega, S. & Grogan, P. Deeper snow enhances winter respiration from both plant-associated and bulk soil carbon pools in birch hummock tundra. Ecosystems 10, 419–431 (2007).
Morgner, E. et al. The importance of winter in annual ecosystem respiration in the high arctic: Effects of snow depth in two vegetation types. Polar Res. 29, 58–74 (2010).
Virtanen, R. Impact of grazing and neighbour removal on a heath plant community transplanted onto a snowbed site, NW Finnish Lapland. Oikos 81, 359–367 (1998).
Choler, P., Michalet, R. & Callaway, R. M. Facilitation and competition on gradients in alpine plant communities. Ecology 82, 3295–3308 (2001).
Eriksson, O. E. The Non-Lichenized Pyrenomycetes of Sweden (Lund, SBT-förlaget, 1992).
Acknowledgements
The Abisko Scientific Research Station provided accommodation, laboratory facilities and funding during the periods of field work. The study was supported by grants from the Centre for Environmental Research in Umeå (CMF) and The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning to J.O. and NER/A/S/2001/00460 from the Natural Environment Research Council, UK, to R.B.
Author information
Authors and Affiliations
Contributions
R.B. initiated the field experiment and managed it together with J.O. S.S. carried out the soil analyses. R.B. and J.O. were together responsible for all other field measurements. M.T. contributed to the field work. J.O., L.E. and R.B. outlined the scope of the study and linked various components. J.O. carried out the statistical analyses and wrote the manuscript, to which all authors contributed with discussions and text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 617 kb)
Rights and permissions
About this article
Cite this article
Olofsson, J., Ericson, L., Torp, M. et al. Carbon balance of Arctic tundra under increased snow cover mediated by a plant pathogen. Nature Clim Change 1, 220–223 (2011). https://doi.org/10.1038/nclimate1142
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1142
This article is cited by
-
Thirty-year dryland crop rotation improves soil multifunctionality and shifts soil fungal community
Plant and Soil (2023)
-
Immediate and legacy effects of snow exclusion on soil fungal diversity and community composition
Forest Ecosystems (2021)
-
Long term crop rotation effect on subsequent soybean yield explained by soil and root-associated microbiomes and soil health indicators
Scientific Reports (2021)
-
Crowberry (Empetrum): A Chief Arctic Traditional Indigenous Fruit in Need of Economic and Ecological Management
The Botanical Review (2021)
-
Arctic plants threatened by winter snow loss
Nature Climate Change (2018)