Abstract
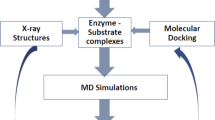
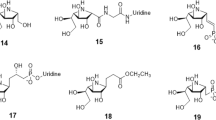
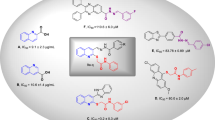
Enzyme inhibition through mimicry of the transition state is a major area for the design of new therapeutic agents. Emerging evidence suggests that many retaining glycosidases that are active on α- or β-mannosides harness unusual B2,5 (boat) transition states. Here we present the analysis of 25 putative β-mannosidase inhibitors, whose Ki values range from nanomolar to millimolar, on the Bacteroides thetaiotaomicron β-mannosidase BtMan2A. B2,5 or closely related conformations were observed for all tightly binding compounds. Subsequent linear free energy relationships that correlate log Ki with log Km/kcat for a series of active center variants highlight aryl-substituted mannoimidazoles as powerful transition state mimics in which the binding energy of the aryl group enhances both binding and the degree of transition state mimicry. Support for a B2,5 transition state during enzymatic β-mannosidase hydrolysis should also facilitate the design and exploitation of transition state mimics for the inhibition of retaining α-mannosidases—an area that is emerging for anticancer therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davies, G.J., Ducros, V.M.-A., Varrot, A. & Zechel, D.L. Mapping the conformational itinerary of β-glycosidases by X-ray crystallography. Biochem. Soc. Trans. 31, 523–527 (2003).
Davies, G., Sinnott, M.L. & Withers, S.G. in Comprehensive Biological Catalysis Vol. 1 (ed. Sinnott, M.L.) 119–209 (Academic Press, London, 1997).
Vasella, A., Davies, G. & Böhm, M. Glycosidase mechanisms. Curr. Opin. Chem. Biol. 6, 619–629 (2002).
Crich, D. & Li, L.F. 4,6-O-benzylidene-directed beta-mannopyranosylation and alpha-glucopyranosylation: the 2-deoxy-2-fluoro and 3-deoxy-3-fluoro series of donors and the importance of the O2–C2-C3–O3 interaction. J. Org. Chem. 72, 1681–1690 (2007).
Crich, D. & Chandrasekera, N.S. Mechanism of 4,6–0-benzylidene-directed beta-mannosylation as determined by alpha-deuterium kinetic isotope effects. Angew. Chem. Int. Ed. 43, 5386–5389 (2004).
Granovsky, M. et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6, 306–312 (2000).
Li, B. et al. Inhibition of Golgi mannosidase II with mannostatin A analogues: synthesis, biological evaluation, and structure-activity relationship studies. ChemBioChem 5, 1220–1227 (2004).
Shah, N., Kuntz, D.A. & Rose, D.R. Comparison of kifunensine and 1-deoxymannojirimycin binding to class I and II alpha-mannosidases demonstrates different saccharide distortions in inverting and retaining catalytic mechanisms. Biochemistry 42, 13812–13816 (2003).
Wen, X., Yuan, Y., Kuntz, D.A., Rose, D.R. & Pinto, B.M. A combined STD-NMR/molecular modeling protocol for predicting the binding modes of the glycosidase inhibitors kifunensine and salacinol to Golgi alpha-mannosidase II. Biochemistry 44, 6729–6737 (2005).
Ducros, V. et al. Substrate distortion by a β-mannanase: snapshots of the Michaelis and covalent intermediate complexes suggest a B2,5 conformation for the transition-state. Angew. Chem. Int. Ed. 41, 2824–2827 (2002).
Stoddart, J.F. Stereochemistry of Carbohydrates (Wiley Interscience, Toronto, 1971).
Walaszek, Z., Horton, D. & Ekiel, I. Conformational studies on aldonolactones by NMR spectroscopy. Conformations of D-glucono-1,5-lactone and D-manno-1,5-lactone in solution. Carbohydr. Res. 106, 193–201 (1982).
Heck, M.P. et al. Cyclic amidine sugars as transition-state analogue inhibitors of glycosidases: potent competitive inhibitors of mannosidases. J. Am. Chem. Soc. 126, 1971–1979 (2004).
Numao, S., Kuntz, D.A., Withers, S.G. & Rose, D.R. Insights into the mechanism of Drosophila melanogaster Golgi alpha-mannosidase II through the structural analysis of covalent reaction intermediates. J. Biol. Chem. 278, 48074–48083 (2003).
Tailford, L.E. et al. Mannose foraging by Bacteroides thetaiotaomicron: structure and specificity of the β-mannosidase, Man2a. J. Biol. Chem. 282, 11291–11299 (2007).
Best, W.M. et al. The synthesis of a carbohydrate-like dihydrooxazine and tetrahydrooxazine as putative inhibitors of glycoside hydrolases: a direct synthesis of isofagomine. Can. J. Chem. 80, 857–865 (2002).
Macdonald, J.M. & Stick, R.V. The synthesis of a D-glucose-like piperidin-2-one: isofagomine lactam. Aust. J. Chem. 57, 449–453 (2004).
Amorim, L. et al. RCM as a tool to freeze conformation of monosaccharides: synthesis of a beta-mannopyranoside mimic adopting a conformation close to the biologically relevant B2,5 boat. Tetrahedr. Lett. 47, 8887–8891 (2006).
Goddard-Borger, E.D. & Stick, R.V. An expeditious synthesis of isofagomine. Aust. J. Chem. 60, 211–213 (2007).
Meloncelli, P.J. & Stick, R.V. Improvements to the synthesis of isofagomine, noeuromycin, azafagomine, and isofagomine lactam, and a synthesis of azanoeuromycin and 'guanidine' isofagomine. Aust. J. Chem. 59, 827–833 (2006).
Terinek, M. & Vasella, A. Synthesis of C(2)-substituted manno-configured tetrahydroimidazopyridines and their evaluation as inhibitors of snail beta-mannosidase. Helv. Chim. Acta 86, 3482–3509 (2003).
Bohm, M., Lorthiois, E., Meyyappan, M. & Vasella, A. Isoquinuclidine mimics of beta-D-glucopyranosides: differences and similarities in the mechanism of action of some beta-D-glucosidases and a beta-D-mannosidase. Helv. Chim. Acta 86, 3818–3835 (2003).
Legler, G. & Julich, E. Synthesis of 5-amino-5-deoxy-D-mannopyranose and 1,5-di-deoxy-1,5-imino-D-mannitol, and inhibition of alpha-D-mannosidases and beta-D-mannosidases. Carbohydr. Res. 128, 61–72 (1984).
Gloster, T.M. et al. Glycosidase inhibition: an assessment of the binding of eighteen putative transition-state mimics. J. Am. Chem. Soc. 129, 2345–2354 (2007).
Gloster, T.M. et al. Structural, kinetic and thermodynamic analysis of glucoimidazole-derived glycosidase inhibitors. Biochemistry 45, 11879–11884 (2006).
Wicki, J., Williams, S.J. & Withers, S.G. Transition-state mimicry by glycosidase inhibitors: a critical kinetic analysis. J. Am. Chem. Soc. 129, 4530–4531 (2007).
Zechel, D.L. et al. Iminosugar glycosidase inhibitors: structural and thermodynamic dissection of the binding of isofagomine and 1-deoxynojirimycin to two β-glucosidases. J. Am. Chem. Soc. 125, 14313–14323 (2003).
Zechel, D.L. et al. Mechanism, mutagenesis, and chemical rescue of beta-mannosidase from Cellulomonas fimi. Biochemistry 42, 7195–7204 (2003).
Capon, B. Mechanism in carbohydrate chemistry. Chem. Rev. 69, 407–498 (1969).
Vincent, F. et al. Common inhibition of both β-glucosidase and β-mannosidase by isofagomine lactam reflects different conformational itineraries for pyranoside hydrolysis. ChemBioChem 5, 1596–1599 (2004).
Money, V.A. et al. Substrate distortion by a lichenase highlights the different conformational itineraries harnessed by related glycoside hydrolases. Angew. Chem. Int. Ed. 45, 5136–5140 (2006).
Bartlett, P.A. & Marlowe, C.K. Phosphonamidates as transition-state analog inhibitors of thermolysin. Biochemistry 22, 4618–4624 (1983).
Whitworth, G.E. et al. Analysis of PUGNAc and NAG-thiazoline as transition state analogues for human O-GlcNAcase: structural and mechanistic insights into inhibitor selectivity and transition state poise. J. Am. Chem. Soc. 129, 635–644 (2007).
Berland, C.R., Sigurskjold, B.W., Stoffer, B., Frandsen, T.P. & Svensson, B. Thermodynamics of inhibitor binding to mutant forms of glucoamylase from Aspergillus niger determined by isothermal titration calorimetry. Biochemistry 34, 10153–10161 (1995).
Withers, S.G., Namchuk, M. & Mosi, R. in Iminosugars As Glycosidase Inhibitors. Nojirimycin and Beyond (ed. Stütz, A.E.) 188–206 (Wiley-VCH, Weinheim, Germany, 1999).
Mosi, R. et al. Reassessment of acarbose as a transition state analogue inhibitor of cyclodextrin glycosyltransferase. Biochemistry 37, 17192–17198 (1998).
Mader, M.M. & Bartlett, P.A. Binding energy and catalysis: the implications for transition state analogs and catalytic antibodies. Chem. Rev. 97, 1281–1301 (1997).
Liu, H.Z., Liang, X.F., Søhoel, H., Bülow, A. & Bols, M. Noeuromycin, a glycosyl cation mimic that strongly inhibits glycosidases. J. Am. Chem. Soc. 123, 5116–5117 (2001).
Tong, M.K., Papandreou, G. & Ganem, B. Potent, broad-spectrum inhibition of glycosidases by an amidine derivative of D-glucose. J. Am. Chem. Soc. 112, 6137–6139 (1990).
Papandreou, G., Tong, M.K. & Ganem, B. Amidine, amidrazone, and amidoxime derivatives of monosaccharide aldonolactams: synthesis and evaluation as glycosidase inhibitors. J. Am. Chem. Soc. 115, 11682–11690 (1993).
Hoos, R. et al. D-Gluconhydroximo-1,5-lactam and related N-arylcarbamates - theoretical calculations, structure, synthesis, and inhibitory effect on beta-glucosidases. Helv. Chim. Acta 76, 2666–2686 (1993).
Snider, M.J. & Wolfenden, R. Site-bound water and the shortcomings of a less than perfect transition state analogue. Biochemistry 40, 11364–11371 (2001).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Esnouf, R.M. An extensively modified version of MolScript that includes greatly enhanced coloring capabilities. J. Mol. Graph. Model. 15, 132–134 (1997).
Acknowledgements
The Biotechnology and Biological Sciences Research Council (BBSRC) is thanked for funding. G.J.D. is the recipient of a Royal Society-Wolfson Research Merit Award. S. Withers (University of British Columbia) is thanked for provision of 4-nitrophenyl 2-deoxy-β-D-arabino-hexopyranoside. A.V. thanks the Swiss National Science Foundation, Hoffmann-La Roche (Basle) and Syngenta (Basle) for generous support. This work is dedicated by M.-P.H., A.V. and J.G. to the memory of C. Mioskowski (deceased on June 2, 2007).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3 (PDF 68 kb)
Rights and permissions
About this article
Cite this article
Tailford, L., Offen, W., Smith, N. et al. Structural and biochemical evidence for a boat-like transition state in β-mannosidases. Nat Chem Biol 4, 306–312 (2008). https://doi.org/10.1038/nchembio.81
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.81
This article is cited by
-
Microbial β-mannosidases and their industrial applications
Applied Microbiology and Biotechnology (2019)
-
Inhibition of a bacterial O-GlcNAcase homologue by lactone and lactam derivatives: structural, kinetic and thermodynamic analyses
Amino Acids (2011)
-
Mechanistic insights into a Ca2+-dependent family of α-mannosidases in a human gut symbiont
Nature Chemical Biology (2010)
-
β-Mannoside hydrolysis goes by boat
Nature Chemical Biology (2008)