Abstract
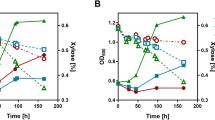
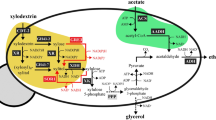
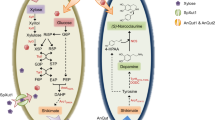
Efficient substrate utilization is the first and most important prerequisite for economically viable production of biofuels and chemicals by microbial cell factories. However, production rates and yields are often compromised by low transport rates of substrates across biological membranes and their diversion to competing pathways. This is especially true when common chassis organisms are engineered to utilize nonphysiological feedstocks. Here, we addressed this problem by constructing an artificial complex between an endogenous sugar transporter and a heterologous xylose isomerase in Saccharomyces cerevisiae. Direct feeding of the enzyme through the transporter resulted in acceleration of xylose consumption and substantially diminished production of xylitol as an undesired side product, with a concomitant increase in the production of ethanol. This underlying principle could also likely be implemented in other biotechnological applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Srere, P.A. Complexes of sequential metabolic enzymes. Annu. Rev. Biochem. 56, 89–124 (1987).
Srere, P.A. Macromolecular interactions: tracing the roots. Trends Biochem. Sci. 25, 150–153 (2000).
Castellana, M. et al. Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat. Biotechnol. 32, 1011–1018 (2014).
Knighton, D.R. et al. Structure of and kinetic channelling in bifunctional dihydrofolate reductase-thymidylate synthase. Nat. Struct. Biol. 1, 186–194 (1994).
Zhou, Z.H., McCarthy, D.B., O'Connor, C.M., Reed, L.J. & Stoops, J.K. The remarkable structural and functional organization of the eukaryotic pyruvate dehydrogenase complexes. Proc. Natl. Acad. Sci. USA 98, 14802–14807 (2001).
Good, M.C., Zalatan, J.G. & Lim, W.A. Scaffold proteins: hubs for controlling the flow of cellular information. Science 332, 680–686 (2011).
Graham, J.W. et al. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell 19, 3723–3738 (2007).
Araiza-Olivera, D. et al. A glycolytic metabolon in Saccharomyces cerevisiae is stabilized by F-actin. FEBS J. 280, 3887–3905 (2013).
Bauler, P., Huber, G., Leyh, T. & McCammon, J.A. Channeling by proximity: The catalytic advantages of active site colocalization using brownian dynamics. J. Phys. Chem. Lett. 1, 1332–1335 (2010).
Moraes, T.F. & Reithmeier, R.A.F. Membrane transport metabolons. Biochim. Biophys. Acta 1818, 2687–2706 (2012).
Meadow, N.D., Fox, D.K. & Roseman, S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu. Rev. Biochem. 59, 497–542 (1990).
Vince, J.W. & Reithmeier, R.A. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J. Biol. Chem. 273, 28430–28437 (1998).
Sterling, D., Reithmeier, R.A. & Casey, J.R. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 276, 47886–47894 (2001).
Chen, A.H. & Silver, P.A. Designing biological compartmentalization. Trends Cell Biol. 22, 662–670 (2012).
Lee, H., DeLoache, W.C. & Dueber, J.E. Spatial organization of enzymes for metabolic engineering. Metab. Eng. 14, 242–251 (2012).
Ljungcrantz, P. et al. Construction of an artificial bifunctional enzyme, beta-galactosidase/galactose dehydrogenase, exhibiting efficient galactose channeling. Biochemistry 28, 8786–8792 (1989).
Tian, L. & Dixon, R.A. Engineering isoflavone metabolism with an artificial bifunctional enzyme. Planta 224, 496–507 (2006).
Iturrate, L., Sánchez-Moreno, I., Oroz-Guinea, I., Pérez-Gil, J. & García-Junceda, E. Preparation and characterization of a bifunctional aldolase/kinase enzyme: a more efficient biocatalyst for C-C bond formation. Chemistry 16, 4018–4030 (2010).
Kirby, J. et al. Enhancing Terpene yield from sugars via novel routes to 1-deoxy-D-xylulose 5-phosphate. Appl. Environ. Microbiol. 81, 130–138 (2015).
Dueber, J.E. et al. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 27, 753–759 (2009).
Oreb, M., Dietz, H., Farwick, A. & Boles, E. Novel strategies to improve co-fermentation of pentoses with D-glucose by recombinant yeast strains in lignocellulosic hydrolysates. Bioengineered 3, 347–351 (2012).
Kötter, P., Amore, R., Hollenberg, C.P. & Ciriacy, M. Isolation and characterization of the Pichia stipitis xylitol dehydrogenase gene, XYL2, and construction of a xylose-utilizing Saccharomyces cerevisiae transformant. Curr. Genet. 18, 493–500 (1990).
Kuyper, M. et al. High-level functional expression of a fungal xylose isomerase: the key to efficient ethanolic fermentation of xylose by Saccharomyces cerevisiae? FEMS Yeast Res. 4, 69–78 (2003).
Brat, D., Boles, E. & Wiedemann, B. Functional expression of a bacterial xylose isomerase in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 75, 2304–2311 (2009).
Demeke, M.M. et al. Combining inhibitor tolerance and D-xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose-based bioethanol production. Biotechnol. Biofuels 6, 120 (2013).
Kuyper, M. et al. Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res. 5, 399–409 (2005).
Demeke, M.M. et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 6, 89 (2013).
Linck, A. et al. On the role of GAPDH isoenzymes during pentose fermentation in engineered Saccharomyces cerevisiae. FEMS Yeast Res. 14, 389–398 (2014).
Tanino, T. et al. Construction of a xylose-metabolizing yeast by genome integration of xylose isomerase gene and investigation of the effect of xylitol on fermentation. Appl. Microbiol. Biotechnol. 88, 1215–1221 (2010).
Asbóth, B. & Náray-Szabó, G. Mechanism of action of D-xylose isomerase. Curr. Protein Pept. Sci. 1, 237–254 (2000).
Farwick, A., Bruder, S., Schadeweg, V., Oreb, M. & Boles, E. ENGINEERING OF YEAST HEXOSE TRANSPORTERS TO TRANSPORT D -xylose without inhibition by D -glucose. Proc. Natl. Acad. Sci. USA 111, 5159–5164 (2014).
Reinke, A.W., Grant, R.A. & Keating, A.E. A synthetic coiled-coil interactome provides heterospecific modules for molecular engineering. J. Am. Chem. Soc. 132, 6025–6031 (2010).
Thompson, K.E., Bashor, C.J., Lim, W.A. & Keating, A.E. SYNZIP protein interaction toolbox: in vitro and in vivo specifications of heterospecific coiled-coil interaction domains. ACS Synth. Biol. 1, 118–129 (2012).
Wang, Y. & Yu, O. Synthetic scaffolds increased resveratrol biosynthesis in engineered yeast cells. J. Biotechnol. 157, 258–260 (2012).
Zettl, M. & Way, M. The WH1 and EVH1 domains of WASP and Ena/VASP family members bind distinct sequence motifs. Curr. Biol. 12, 1617–1622 (2002).
Karim, A.S., Curran, K.A. & Alper, H.S. Characterization of plasmid burden and copy number in Saccharomyces cerevisiae for optimization of metabolic engineering applications. FEMS Yeast Res. 13, 107–116 (2013).
Roy, A., Dement, A.D., Cho, K.H. & Kim, J.-H. Assessing glucose uptake through the yeast hexose transporter 1 (Hxt1). PLoS One 10, e0121985 (2015).
Wieczorke, R., Dlugai, S., Krampe, S. & Boles, E. Characterisation of mammalian GLUT glucose transporters in a heterologous yeast expression system. Cell. Physiol. Biochem. 13, 123–134 (2003).
Smirnova, I. et al. Outward-facing conformers of LacY stabilized by nanobodies. Proc. Natl. Acad. Sci. USA 111, 18548–18553 (2014).
Matsushika, A., Inoue, H., Kodaki, T. & Sawayama, S. Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives. Appl. Microbiol. Biotechnol. 84, 37–53 (2009).
Sowah, D. & Casey, J.R. An intramolecular transport metabolon: fusion of carbonic anhydrase II to the COOH terminus of the Cl(-)/HCO(3)(-)exchanger, AE1. Am. J. Physiol. Cell Physiol. 301, C336–C346 (2011).
Kuyper, M. et al. Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res. 5, 925–934 (2005).
Aguilera, J. & Prieto, J.A. The Saccharomyces cerevisiae aldose reductase is implied in the metabolism of methylglyoxal in response to stress conditions. Curr. Genet. 39, 273–283 (2001).
Wieczorke, R. et al. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464, 123–128 (1999).
Gietz, R.D. & Schiestl, R.H. Frozen competent yeast cells that can be transformed with high efficiency using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 1–4 (2007).
Bruder, S., Reifenrath, M., Thomik, T., Boles, E. & Herzog, K. Parallelised online biomass monitoring in shake flasks enables efficient strain and carbon source dependent growth characterisation of Saccharomyces cerevisiae. Microb. Cell Fact. 15, 127 (2016).
Oldenburg, K.R., Vo, K.T., Michaelis, S. & Paddon, C. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 25, 451–452 (1997).
Brat, D., Weber, C., Lorenzen, W., Bode, H.B. & Boles, E. Cytosolic re-localization and optimization of valine synthesis and catabolism enables increased isobutanol production with the yeast Saccharomyces cerevisiae. Biotechnol. Biofuels 5, 65 (2012).
Horak, J., Regelmann, J. & Wolf, D.H. Two distinct proteolytic systems responsible for glucose-induced degradation of fructose-1,6-bisphosphatase and the Gal2p transporter in the yeast Saccharomyces cerevisiae share the same protein components of the glucose signaling pathway. J. Biol. Chem. 277, 8248–8254 (2002).
Iancu, C.V., Zamoon, J., Woo, S.B., Aleshin, A. & Choe, J.Y. Crystal structure of a glucose/H+ symporter and its mechanism of action. Proc. Natl. Acad. Sci. USA 110, 17862–17867 (2013).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Acknowledgements
Financial support by German Federal Ministry of Food, Agriculture and Consumer Protection following a decision of German Bundestag (to E.B. and M.O.; project ECO-FERM, FKZ: 22031811) is gratefully acknowledged. We would like to thank D. Wolf (University of Stuttgart) for kindly providing Gal2 antibodies. We are grateful to C. Hiesl, C. Hannig and S. Scholl for their contribution to the experimental work. We thank J. Tripp and W. Generoso for stimulating discussions. T. Bionda is acknowledged for critical comments on the manuscript.
Author information
Authors and Affiliations
Contributions
M.O. conceived and supervised the project. E.B. co-supervised the project and provided resources. T.T., I.W., E.B. and M.O. contributed to experimental design. T.T. performed the experiments. T.T. and M.O. analyzed the data. J.C. performed structure modeling. M.O. wrote the manuscript, which was edited and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1 and 2, and Supplementary Figures 1–8 (PDF 1648 kb)
Rights and permissions
About this article
Cite this article
Thomik, T., Wittig, I., Choe, Jy. et al. An artificial transport metabolon facilitates improved substrate utilization in yeast. Nat Chem Biol 13, 1158–1163 (2017). https://doi.org/10.1038/nchembio.2457
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2457
This article is cited by
-
Microbial synthesis of long-chain α-alkenes from methanol by engineering Pichia pastoris
Bioresources and Bioprocessing (2022)
-
Enzyme assemblies on demand
Nature Chemical Biology (2022)
-
Optimal spatial allocation of enzymes as an investment problem
Communications Physics (2022)
-
Genetic fusion of P450 BM3 and formate dehydrogenase towards self-sufficient biocatalysts with enhanced activity
Scientific Reports (2021)
-
An integrative approach to improving the biocatalytic reactions of whole cells expressing recombinant enzymes
World Journal of Microbiology and Biotechnology (2021)