Abstract
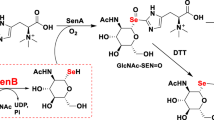
Sulfoquinovose is produced by photosynthetic organisms at a rate of 1010 tons per annum and is degraded by bacteria as a source of carbon and sulfur. We have identified Escherichia coli YihQ as the first dedicated sulfoquinovosidase and the gateway enzyme to sulfoglycolytic pathways. Structural and mutagenesis studies unveiled the sequence signatures for binding the distinguishing sulfonate residue and revealed that sulfoquinovoside degradation is widespread across the tree of life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Harwood, J.L. & Nicholls, R.G. Biochem. Soc. Trans. 7, 440–447 (1979).
Benning, C. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 53–75 (1998).
Denger, K. et al. Nature 507, 114–117 (2014).
Felux, A.K., Spiteller, D., Klebensberger, J. & Schleheck, D. Proc. Natl. Acad. Sci. USA 112, E4298–E4305 (2015).
Shimojima, M. Prog. Lipid Res. 50, 234–239 (2011).
Martelli, H.L. & Benson, A.A. Biochim. Biophys. Acta 93, 169–171 (1964).
Roy, A.B., Hewlins, M.J., Ellis, A.J., Harwood, J.L. & White, G.F. Appl. Environ. Microbiol. 69, 6434–6441 (2003).
Denger, K., Huhn, T., Hollemeyer, K., Schleheck, D. & Cook, A.M. FEMS Microbiol. Lett. 328, 39–45 (2012).
Sugimoto, K., Sato, N. & Tsuzuki, M. FEBS Lett. 581, 4519–4522 (2007).
Durham, B.P. et al. Proc. Natl. Acad. Sci. USA 112, 453–457 (2015).
Shibuya, I. & Benson, A.A. Nature 192, 1186–1187 (1961).
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P.M. & Henrissat, B. Nucleic Acids Res. 42, D490–D495 (2014).
Okuyama, M., Mori, H., Chiba, S. & Kimura, A. Protein Expr. Purif. 37, 170–179 (2004).
Andersson, L., Carriére, F., Lowe, M.E., Nilsson, A. & Verger, R. Biochim. Biophys. Acta 1302, 236–240 (1996).
Lee, S.S., Yu, S. & Withers, S.G. J. Am. Chem. Soc. 124, 4948–4949 (2002).
McCarter, J.D. & Withers, S.G. J. Am. Chem. Soc. 118, 241–242 (1996).
Quaroni, A. & Semenza, G. J. Biol. Chem. 251, 3250–3253 (1976).
Okuyama, M. et al. Eur. J. Biochem. 268, 2270–2280 (2001).
Davies, G.J., Planas, A. & Rovira, C. Acc. Chem. Res. 45, 308–316 (2012).
Speciale, G., Thompson, A.J., Davies, G.J. & Williams, S.J. Curr. Opin. Struct. Biol. 28, 1–13 (2014).
Tagami, T. et al. J. Biol. Chem. 288, 19296–19303 (2013).
Studier, F.W. Protein Expr. Purif. 41, 207–234 (2005).
Winter, G. J. Appl. Cryst. 43, 186–190 (2010).
Collaborative Computational Project, Number 4. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Celniker, G. et al. Isr. J. Chem. 53, 199–206 (2013).
Ashkenazy, H., Erez, E., Martz, E., Pupko, T. & Ben-Tal, N. Nucleic Acids Res. 38, W529–W533 (2010).
The PyMOL Molecular Graphics System version 1.7.4 (Schrödinger, LLC).
McNicholas, S., Potterton, E., Wilson, K.S. & Noble, M.E.M. Acta Crystallogr. D Biol. Crystallogr. 67, 386–394 (2011).
Dereeper, A. et al. Nucleic Acids Res. 36, W465–W469 (2008).
Acknowledgements
We thank S.G. Withers for the gift of 5-fluoro-β-L-idopyranosyl fluoride and N.A. Williamson for technical assistance. This work was supported by grants from the UK Biotechnology and Biological Sciences Research Council and the European Research Council (AdG-322942 to G.J.D.), the Australian Research Council (to S.J.W.), the Ramaciotti Foundation and the Victorian Endowment for Science Knowledge and Innovation, with additional support from the Australian Cancer Research Foundation and Victorian State Government Operational Infrastructure Support, NHMRC IRIISS grant 9000220 (to E.D.G.-B.). We thank the Diamond Light Source (Didcot, UK) for access to beamlines IO4, IO4-1 and IO2 (proposal number mx-9948).
Author information
Authors and Affiliations
Contributions
G.S. synthesized substrate and performed LC/MS analysis. G.S. and E.D.G.-B. cloned, expressed, mutagenized and purified enzymes and performed kinetic analyses. Y.J. performed crystallographic studies and prepared the accompanying figures. Experiments were designed by G.J.D., S.J.W. and E.D.G.-B., who collectively wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–3 and Supplementary Figures 1–7. (PDF 2820 kb)
Supplementary Note
Synthetic Procedures (PDF 529 kb)
Rights and permissions
About this article
Cite this article
Speciale, G., Jin, Y., Davies, G. et al. YihQ is a sulfoquinovosidase that cleaves sulfoquinovosyl diacylglyceride sulfolipids. Nat Chem Biol 12, 215–217 (2016). https://doi.org/10.1038/nchembio.2023
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2023
This article is cited by
-
Insight into broad substrate specificity and synergistic contribution of a fungal α-glucosidase in Chinese Nong-flavor daqu
Microbial Cell Factories (2023)
-
Genome sequences of Arthrobacter spp. that use a modified sulfoglycolytic Embden–Meyerhof–Parnas pathway
Archives of Microbiology (2022)
-
Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut
The ISME Journal (2021)
-
Sulfur metabolites in the pelagic ocean
Nature Reviews Microbiology (2019)
-
Operational Experience of an Open-Access, Subscription-Based Mass Spectrometry and Proteomics Facility
Journal of the American Society for Mass Spectrometry (2018)