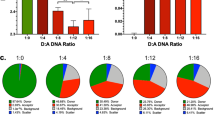
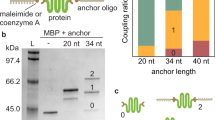
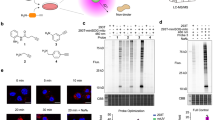
Abstract
Recombinant polypeptides and protein domains containing two cysteine pairs located distal in primary sequence but proximal in the native folded or assembled state are labeled selectively in vitro and in mammalian cells using the profluorescent biarsenical reagents FlAsH-EDT2 and ReAsH-EDT2. This strategy, termed bipartite tetracysteine display, enables the detection of protein-protein interactions and alternative protein conformations in live cells. As proof of principle, we show that the equilibrium stability and fluorescence intensity of polypeptide–biarsenical complexes correlates with the thermodynamic stability of the protein fold or assembly. Destabilized protein variants form less stable and less bright biarsenical complexes, which allows discrimination of live cells expressing folded polypeptide and protein domains from those containing disruptive point mutations. Bipartite tetracysteine display may provide a means to detect early protein misfolding events associated with Alzheimer's disease, Parkinson's disease and cystic fibrosis; it may also enable high-throughput screening of compounds that stabilize discrete protein folds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shimomura, O., Johnson, F.H. & Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 59, 223–239 (1962).
Prasher, D.C., Eckenrode, V.K., Ward, W.W., Prendergast, F.G. & Cormier, M.J. Primary structure of the Aequorea victoria green fluorescent protein. Gene 111, 229–233 (1992).
Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Giepmans, B.N., Adams, S.R., Ellisman, M.H. & Tsien, R.Y. The fluorescent toolbox for assessing protein location and function. Science 312, 217–224 (2006).
Chudakov, D.M., Lukyanov, S. & Lukyanov, K.A. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 23, 605–613 (2005).
Willemse, M., Janssen, E., de Lange, F., Wieringa, B. & Fransen, J. ATP and FRET – a cautionary note. Nat. Biotechnol. 25, 170–172 (2007).
Lippincott-Schwartz, J. & Patterson, G.H. Development and use of fluorescent protein markers in living cells. Science 300, 87–91 (2003).
Griffin, B.A., Adams, S.R. & Tsien, R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science 281, 269–272 (1998).
Martin, B.R., Giepmans, B.N.G., Adams, S.R. & Tsien, R.Y. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 23, 1308–1314 (2005).
Gaietta, G.M. et al. Golgi twins in late mitosis revealed by genetically encoded tags for live cell imaging and correlated electron microscopy. Proc. Natl. Acad. Sci. USA 103, 17777–17782 (2006).
Nakanishi, J., Takarada, T., Yunoki, S., Kikuchi, Y. & Maeda, M. FRET-based monitoring of conformational change of the β2 adrenergic receptor in living cells. Biochem. Biophys. Res. Commun. 343, 1191–1196 (2006).
Hoffmann, C. et al. A FlAsH-based FRET approach to determine G protein - coupled receptor activation in living cells. Nat. Methods 2, 171–176 (2005).
Adams, S.R. et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 124, 6063–6076 (2002).
Andresen, M., Schmitz-Salue, R. & Jakobs, S. Short tetracysteine tags to β-tubulin demonstrate the significance of small labels for live cell imaging. Mol. Biol. Cell 15, 5616–5622 (2004).
Dyachok, O., Isakov, Y., Sagetorp, J. & Tengholm, A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 439, 349–352 (2006).
Enninga, J., Mounier, J., Sansonetti, P. & Tran Van Nhieu, G. Secretion of type III effectors into host cells in real time. Nat. Methods 2, 959–965 (2005).
Ignatova, Z. & Gierasch, L.M. Monitoring protein stability and aggregation in vivo by real-time fluorescent labeling. Proc. Natl. Acad. Sci. USA 101, 523–528 (2004).
Ignatova, Z. & Gierasch, L.M. Aggregation of a slow-folding mutant of a beta-clam protein proceeds through a monomeric nucleus. Biochemistry 44, 7266–7274 (2005).
Ignatova, Z. & Gierasch, L.M. Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant. Proc. Natl. Acad. Sci. USA 103, 13357–13361 (2006).
Blundell, T.L., Pitts, J.E., Tickle, I.J., Wood, S.P. & Wu, C.W. X-ray analysis (1.4 Å resolution) of avian pancreatic-polypeptide - small globular protein hormone. Proc. Natl. Acad. Sci. USA 78, 4175–4179 (1981).
Cochran, A.G., Skelton, N.J. & Starovasnik, M.A. Tryptophan zippers: stable, monomeric β-hairpins. Proc. Natl. Acad. Sci. USA 98, 5578–5583 (2001).
O'Shea, E.K., Klemm, J.D., Kim, P.S. & Alber, T. X-ray structure of the GCN4 leucine zipper, a 2-stranded, parallel coiled coil. Science 254, 539–544 (1991).
Junius, F.K., O'Donoghue, S.I., Nilges, M., Weiss, A.S. & King, G.F. High resolution NMR solution structure of the leucine zipper domain of the c-Jun homodimer. J. Biol. Chem. 271, 13663–13667 (1996).
Watt, R.M. & Voss, E.W., Jr. Mechanism of quenching of fluorescein by anti-fluorescein IgG antibodies. Immunochemistry 14, 533–551 (1977).
Sreerama, N., Venyaminov, S.Y. & Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: inclusion of denatured proteins with native proteins in the analysis. Anal. Biochem. 287, 243–251 (2000).
Hammarstrom, P., Wiseman, R.L., Powers, E.T. & Kelly, J.W. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science 299, 713–716 (2003).
Schuler, B., Lipman, E.A., Steinbach, P.J., Kumke, M. & Eaton, W.A. Polyproline and the “spectroscopic ruler” revisited with single-molecule fluorescence. Proc. Natl. Acad. Sci. USA 102, 2754–2759 (2005).
Acknowledgements
This work was supported in part by the US National Institutes of Health and in part by a grant to Yale University, in support of A.S., from the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
N.W.L. contributed to experimental design, fluorophore synthesis, in vitro binding analyses, CD and manuscript drafting; R.J.D. contributed to experimental design and live cell experiments; D.B.F. contributed to in vitro binding analyses, CD and characterization of polypeptide-FlAsH complexes; A.S. contributed to experimental design and manuscript drafting.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1–3, and Supplementary Methods (PDF 694 kb)
Rights and permissions
About this article
Cite this article
Luedtke, N., Dexter, R., Fried, D. et al. Surveying polypeptide and protein domain conformation and association with FlAsH and ReAsH. Nat Chem Biol 3, 779–784 (2007). https://doi.org/10.1038/nchembio.2007.49
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2007.49
This article is cited by
-
Identification of transmissible proteotoxic oligomer-like fibrils that expand conformational diversity of amyloid assemblies
Communications Biology (2021)
-
In vivo induction of membrane damage by β-amyloid peptide oligomers
Acta Neuropathologica Communications (2018)
-
Characterization of Fluorescein Arsenical Hairpin (FlAsH) as a Probe for Single-Molecule Fluorescence Spectroscopy
Scientific Reports (2017)
-
Advances in chemical labeling of proteins in living cells
Cell and Tissue Research (2015)
-
Amyloid-β forms fibrils by nucleated conformational conversion of oligomers
Nature Chemical Biology (2011)