Abstract
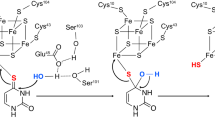
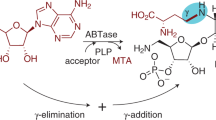
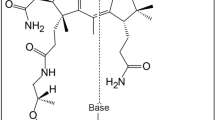
The physiological function for thiaminase II, a thiamin-degrading enzyme, has eluded investigators for more than 50 years. Here, we demonstrate that this enzyme is involved in the regeneration of the thiamin pyrimidine rather than in thiamin degradation, and we identify a new pathway involved in the salvage of base-degraded forms of thiamin. This pathway is widely distributed among bacteria, archaea and eukaryotes. In this pathway, thiamin hydrolysis products such as N-formyl-4-amino-5-aminomethyl-2-methylpyrimidine (formylaminopyrimidine; 15) are transported into the cell using the ThiXYZ transport system, deformylated by the ylmB-encoded amidohydrolase and hydrolyzed to 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP; 6)—an intermediate on the de novo thiamin biosynthetic pathway. To our knowledge this is the first example of a thiamin salvage pathway involving thiamin analogs generated by degradation of one of the heterocyclic rings of the cofactor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Begley, T.P. & Ealick, S.E. Mechanistic and structural studies on thiamine biosynthetic enzymes. Oxidative Stress and Disease 11, 15–28 (2004).
Begley, T.P. Cofactor biosynthesis: an organic chemist's treasure trove. Nat. Prod. Rep. 23, 15–25 (2006).
Lawhorn, B.G., Mehl, R.A. & Begley, T.P. Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction. Org. Biomol. Chem. 2, 2538–2546 (2004).
Park, J.-H., Burns, K., Kinsland, C. & Begley, T.P. Characterization of two kinases involved in thiamine pyrophosphate and pyridoxal phosphate biosynthesis in Bacillus subtilis: 4-amino-5-hydroxymethyl-2-methylpyrimidine kinase and pyridoxal kinase. J. Bacteriol. 186, 1571–1573 (2004).
Dorrestein, P.C., Zhai, H., McLafferty, F.W. & Begley, T.P. The biosynthesis of the thiazole phosphate moiety of thiamin: the sulfur transfer mediated by the sulfur carrier protein ThiS. Chem. Biol. 11, 1373–1381 (2004).
Hanes, J.W., Ealick, S.E. & Begley, T.P. Thiamin phosphate synthase: the rate of pyrimidine carbocation formation. J. Am. Chem. Soc. 129, 4860–4861 (2007).
Webb, E. & Downs, D. Characterization of thiL, encoding thiamin-monophosphate kinase, in Salmonella typhimurium. J. Biol. Chem. 272, 15702–15707 (1997).
Toms, A.V., Haas, A.L., Park, J.-H., Begley, T.P. & Ealick, S.E. Structural characterization of the regulatory proteins TenA and TenI from Bacillus subtilis and identification of TenA as a thiaminase II. Biochemistry 44, 2319–2329 (2005).
Haas, A.L., Laun, N.P. & Begley, T.P. Thi20, a remarkable enzyme from Saccharomyces cerevisiae with dual thiamin biosynthetic and degradation activities. Bioorg. Chem. 33, 338–344 (2005).
Rodionov, D.A., Vitreschak, A.G., Mironov, A.A. & Gelfand, M.S. Comparative genomics of thiamin biosynthesis in procaryotes. J. Biol. Chem. 277, 48949–48959 (2002).
Winkler, W., Nahvi, A. & Breaker, R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419, 952–956 (2002).
Miranda-Rios, J. The THI-box riboswitch, or how RNA binds thiamin pyrophosphate. Structure 15, 259–265 (2007).
Takami, H. & Horikoshi, K. Reidentification of facultatively alkaliphilic Bacillus sp. C-125 to Bacillus halodurans. Biosci. Biotechnol. Biochem. 63, 943–945 (1999).
Horikoshi, K. Alkaliphiles: some applications of their products for biotechnology. Microbiol. Mol. Biol. Rev. 63, 735–750 (1999).
Maier, G.D. & Metzler, D.E. Structures of thiamine in basic solution. J. Am. Chem. Soc. 79, 4386–4391 (1957).
Chahine, E.H. & Dubois, J.M. Kinetics and thermodynamics of the structural transformations of thiamine in neutral and basic aqueous media. The UV spectrum of the tetrahedral pseudobase intermediate. J. Am. Chem. Soc. 105, 2335–2340 (1983).
Xu, J.C., Stucki, J.W., Wu, J., Kostka, J.E. & Sims, G.K. Fate of atrazine and alachlor in redox-treated ferruginous smectite. Environ. Toxicol. Chem. 20, 2717–2724 (2001).
Quayle, J.R. Formate dehydrogenase. Methods Enzymol. 9, 360–364 (1966).
Day, N. & Keillor, J.W. A continuous spectrophotometric linked enzyme assay for transglutaminase activity. Anal. Biochem. 274, 141–144 (1999).
Kurata, G.-I., Sakai, T. & Miyahara, T. Antagonists of thiamine XVIII: reaction condition in the formation of desthiothiamine from alkaline thiamine solution with amino acids. Bitamin 37, 398–402 (1968).
Melnick, J. et al. Identification of the two missing bacterial genes involved in thiamine salvage: thiamine pyrophosphokinase and thiamine kinase. J. Bacteriol. 186, 3660–3662 (2004).
Imamura, N. & Nakayama, H. thiD locus of Escherichia coli. Experientia 37, 1265–1266 (1981).
Imamura, N. & Nakayama, H. thiK and thiL loci of Escherichia coli. J. Bacteriol. 151, 708–717 (1982).
Mizote, T. & Nakayama, H. The thiM locus and its relation to phosphorylation of hydroxyethylthiazole in Escherichia coli. J. Bacteriol. 171, 3228–3232 (1989).
Dornow, A. & Petsch, G. Reductions with lithium aluminum hydride. V. The preparation of vitamin B1. Chem. Ber. 86, 1404–1407 (1953).
Pace, C.N., Vajdos, F., Fee, L., Grimsley, G. & Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4, 2411–2423 (1995).
Anderson, K.S., Sikorski, J.A. & Johnson, K.A. Evalutaion of 5-enolpyruvoylshikimate-3-phosphate synthase substrate and inhibitor binding by stopped-flow and equilibrium fluorescence measurements. Biochemistry 27, 1604–1610 (1988).
Schyns, G. et al. Isolation and characterization of new thiamine-deregulated mutants of Bacillus subtilis. J. Bacteriol. 187, 8127–8136 (2005).
Wach, A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12, 259–265 (1996).
Acknowledgements
We would like to thank C. Kinsland (Protein Purification Facility, Cornell University) for overexpressing TenA and J. Hanes for his help with the Kd determination. We would also like to thank Roche for providing aminopyrimidine. This research was supported by a grant from the US National Institutes of Health (DK44083).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 351 kb)
Rights and permissions
About this article
Cite this article
Jenkins, A., Schyns, G., Potot, S. et al. A new thiamin salvage pathway. Nat Chem Biol 3, 492–497 (2007). https://doi.org/10.1038/nchembio.2007.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2007.13
This article is cited by
-
Dietary factors potentially impacting thiaminase I-mediated thiamine deficiency
Scientific Reports (2023)
-
Evolutionary and ecological correlates of thiaminase in fishes
Scientific Reports (2023)
-
A Possible Aquatic Origin of the Thiaminase TenA of the Human Gut Symbiont Bacteroides thetaiotaomicron
Journal of Molecular Evolution (2023)
-
Thiamine: a key nutrient for yeasts during wine alcoholic fermentation
Applied Microbiology and Biotechnology (2021)
-
Genome-wide Phenotypic Profiling Identifies and Categorizes Genes Required for Mycobacterial Low Iron Fitness
Scientific Reports (2019)