Abstract
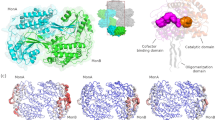
Aldehyde oxidase (AOX) is a xanthine oxidase (XO)-related enzyme with emerging importance due to its role in the metabolism of drugs and xenobiotics. We report the first crystal structures of human AOX1, substrate free (2.6-Å resolution) and in complex with the substrate phthalazine and the inhibitor thioridazine (2.7-Å resolution). Analysis of the protein active site combined with steady-state kinetic studies highlight the unique features, including binding and substrate orientation at the active site, that characterize human AOX1 as an important drug-metabolizing enzyme. Structural analysis of the complex with the noncompetitive inhibitor thioridazine revealed a new, unexpected and fully occupied inhibitor-binding site that is structurally conserved among mammalian AOXs and XO. The new structural insights into the catalytic and inhibition mechanisms of human AOX that we now report will be of great value for the rational analysis of clinical drug interactions involving inhibition of AOX1 and for the prediction and design of AOX-stable putative drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Garattini, E., Fratelli, M. & Terao, M. The mammalian aldehyde oxidase gene family. Hum. Genomics 4, 119–130 (2009).
Garattini, E., Fratelli, M. & Terao, M. Mammalian aldehyde oxidases: genetics, evolution and biochemistry. Cell. Mol. Life Sci. 65, 1019–1048 (2008).
Hille, R., Hall, J. & Basu, P. The mononuclear molybdenum enzymes. Chem. Rev. 114, 3963–4038 (2014).
Garattini, E., Mendel, R., Romao, M.J., Wright, R. & Terao, M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem. J. 372, 15–32 (2003).
Mahro, M. et al. Identification of crucial amino acids in mouse aldehyde oxidase 3 that determine substrate specificity. PLoS ONE 8, e82285 (2013).
Wahl, R.C. & Rajagopalan, K.V. Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J. Biol. Chem. 257, 1354–1359 (1982).
Okamoto, K. et al. The crystal structure of xanthine oxidoreductase during catalysis: implications for reaction mechanism and enzyme inhibition. Proc. Natl. Acad. Sci. USA 101, 7931–7936 (2004).
Jones, R.M., Inscore, F.E., Hille, R. & Kirk, M.L. Freeze-quench magnetic circular dichroism spectroscopic study of the “very rapid” intermediate in xanthine oxidase. Inorg. Chem. 38, 4963–4970 (1999).
Terao, M. et al. Avian and canine aldehyde oxidases. Novel insights into the biology and evolution of molybdo-flavoenzymes. J. Biol. Chem. 281, 19748–19761 (2006).
Garattini, E. & Terao, M. The role of aldehyde oxidase in drug metabolism. Expert Opin. Drug Metab. Toxicol. 8, 487–503 (2012).
Obach, R.S., Huynh, P., Allen, M.C. & Beedham, C. Human liver aldehyde oxidase: inhibition by 239 drugs. J. Clin. Pharmacol. 44, 7–19 (2004).
Pryde, D.C. et al. Aldehyde oxidase: an enzyme of emerging importance in drug discovery. J. Med. Chem. 53, 8441–8460 (2010).
Garattini, E. & Terao, M. Increasing recognition of the importance of aldehyde oxidase in drug development and discovery. Drug Metab. Rev. 43, 374–386 (2011).
Kitamura, S., Sugihara, K. & Ohta, S. Drug-metabolizing ability of molybdenum hydroxylases. Drug Metab. Pharmacokinet. 21, 83–98 (2006).
Fu, C. et al. Aldehyde oxidase 1 (AOX1) in human liver cytosols: quantitative characterization of AOX1 expression level and activity relationship. Drug Metab. Dispos. 41, 1797–1804 (2013).
Rashidi, M.R., Smith, J.A., Clarke, S.E. & Beedham, C. In vitro oxidation of famciclovir and 6-deoxypenciclovir by aldehyde oxidase from human, guinea pig, rabbit, and rat liver. Drug Metab. Dispos. 25, 805–813 (1997).
Beedham, C. The role of non-P450 enzymes in drug oxidation. Pharm. World Sci. 19, 255–263 (1997).
Beedham, C. in Drug Metabolism: Towards the Next Millenium (ed. Gooderham, N.J.) 39–52 (IOS Press, 1998).
Coelho, C. et al. The first mammalian aldehyde oxidase crystal structure: insights into substrate specificity. J. Biol. Chem. 287, 40690–40702 (2012).
Mahro, M. et al. Characterization and crystallization of mouse aldehyde oxidase 3: from mouse liver to Escherichia coli heterologous protein expression. Drug Metab. Dispos. 39, 1939–1945 (2011).
Obach, R.S. & Walsky, R.L. Drugs that inhibit oxidation reactions catalyzed by aldehyde oxidase do not inhibit the reductive metabolism of ziprasidone to its major metabolite, S-methyldihydroziprasidone: an in vitro study. J. Clin. Psychopharmacol. 25, 605–608 (2005).
Rani Basu, L., Mazumdar, K., Dutta, N.K., Karak, P. & Dastidar, S.G. Antibacterial property of the antipsychotic agent prochlorperazine, and its synergism with methdilazine. Microbiol. Res. 160, 95–100 (2005).
Amaral, L. & Viveiros, M. Why thioridazine in combination with antibiotics cures extensively drug-resistant Mycobacterium tuberculosis infections. Int. J. Antimicrob. Agents 39, 376–380 (2012).
Christensen, J.B. et al. A comparative analysis of in vitro and in vivo efficacies of the enantiomers of thioridazine and its racemate. PLoS ONE 8, e57493 (2013).
Christensen, J.B., Hendricks, O. & Kristiansen, J. Thioridazine and derivatives thereof for reversing antimicrobial drug resistance. US patent 8,623,864 (2014).
Baral, P.K. et al. Structural basis of prion inhibition by phenothiazine compounds. Structure 22, 291–303 (2014).
Wang, A., Stout, C.D., Zhang, Q. & Johnson, E.F. Contributions of ionic interactions and protein dynamics to cytochrome P450 2D6 (CYP2D6) substrate and inhibitor binding. J. Biol. Chem. 290, 5092–5104 (2015).
Enroth, C., Eger, B.T., Okamoto, K., Nishino, T. & Pai, E.F. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc. Natl. Acad. Sci. USA 97, 10723–10728 (2000).
Ishikita, H., Eger, B.T., Okamoto, K., Nishino, T. & Pai, E.F. Protein conformational gating of enzymatic activity in xanthine oxidoreductase. J. Am. Chem. Soc. 134, 999–1009 (2012).
Schlauderer, F. et al. Structural analysis of phenothiazine derivatives as allosteric inhibitors of the MALT1 paracaspase. Angew. Chem. Int. Ed. Engl. 52, 10384–10387 (2013).
Wilder, P.T. et al. In vitro screening and structural characterization of inhibitors of the S100B–p53 interaction. Int. J. High Throughput Screen. 2010, 109–126 (2010).
Barr, J.T. & Jones, J.P. Inhibition of human liver aldehyde oxidase: implications for potential drug-drug interactions. Drug Metab. Dispos. 39, 2381–2386 (2011).
Romão, M.J. Molybdenum and tungsten enzymes: a crystallographic and mechanistic overview. Dalton Trans. 21, 4053–4068 (2009).
Palmer, T. et al. Involvement of the narJ and mob gene products in the biosynthesis of the molybdoenzyme nitrate reductase in Escherichia coli. Mol. Microbiol. 20, 875–884 (1996).
Temple, C.A., Graf, T.N. & Rajagopalan, K.V. Optimization of expression of human sulfite oxidase and its molybdenum domain. Arch. Biochem. Biophys. 383, 281–287 (2000).
Hartmann, T. et al. The impact of single nucleotide polymorphisms on human aldehyde oxidase. Drug Metab. Dispos. 40, 856–864 (2012).
Leslie, A.G.W. & Powell, H.R. Evolving Methods for Macromolecular Crystallography (eds. Read, R.J. & Sussman, J.L.) 41–51 (Springer, 2007).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 (2006).
McCoy, A.J., Grosse-Kunstleve, R.W., Storoni, L.C. & Read, R.J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 (2005).
Schwarzenbacher, R., Godzik, A., Grzechnik, S.K. & Jaroszewski, L. The importance of alignment accuracy for molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 60, 1229–1236 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Acknowledgements
This work was financially supported by the Fundação para a Ciência e Tecnologia (FCT-MEC) through projects UID/Multi/04378/2013, EXCL/QEQ-COM/0394/2012, PTDC/BIA-PRO/118377/2010 (M.J.R., T.S.-S., C.C.), SFRH/BPD/84581/2012 (C.C.) and DAAD-441.00 (M.J.R., T.S.-S., S.L.) and by Deutsche Forschungsgemeinschaft Grant Le1171/8-1 (S.L.). We thank the I02 staff of the Diamond Light Source (DLS, Didcot, United Kingdom) and X06DA-PXIII staff from the Swiss Light Source (SLS, Paul Scherrer Institut, Villigen, Switzerland) for assistance during data collection. We also thank the staff from beamlines ID14-1, ID29-1 and ID23-1 from the European Synchrotron Radiation Facility (ESRF, Grenoble, France). The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under BioStruct-X (grant agreement no. 283570). We thank R. Hille (University of California, Riverside) for providing bXO for the inhibition experiments and A. Palma (Universidade Nova de Lisboa, Portugal) for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
C.C., T.S.-S. and M.J.R. conceived and designed the crystallization experiments and performed 3D structure determination. A.F., T.H. and S.L. purified the protein. A.F. and S.L. conducted kinetic experiments. C.C., A.F., T.S.-S., S.L. and M.J.R. performed data analysis. C.C., T.S.-S., S.L. and M.J.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1 and 2 and Supplementary Figures 1–5. (PDF 1884 kb)
Rights and permissions
About this article
Cite this article
Coelho, C., Foti, A., Hartmann, T. et al. Structural insights into xenobiotic and inhibitor binding to human aldehyde oxidase. Nat Chem Biol 11, 779–783 (2015). https://doi.org/10.1038/nchembio.1895
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1895
This article is cited by
-
The past decade of Genentech experience in elucidation of novel reaction mechanisms in drug metabolism
Medicinal Chemistry Research (2023)
-
The pyridazine heterocycle in molecular recognition and drug discovery
Medicinal Chemistry Research (2023)
-
Roles of selected non-P450 human oxidoreductase enzymes in protective and toxic effects of chemicals: review and compilation of reactions
Archives of Toxicology (2022)
-
Effects of Phenothiazines on Aldehyde Oxidase Activity Towards Aldehydes and N-Heterocycles: an In Vitro and In Silico Study
European Journal of Drug Metabolism and Pharmacokinetics (2019)
-
Arabidopsis molybdenum cofactor sulfurase ABA3 contributes to anthocyanin accumulation and oxidative stress tolerance in ABA-dependent and independent ways
Scientific Reports (2018)