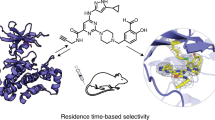
Abstract
Drugs with prolonged on-target residence times often show superior efficacy, yet general strategies for optimizing drug-target residence time are lacking. Here we made progress toward this elusive goal by targeting a noncatalytic cysteine in Bruton's tyrosine kinase (BTK) with reversible covalent inhibitors. Using an inverted orientation of the cysteine-reactive cyanoacrylamide electrophile, we identified potent and selective BTK inhibitors that demonstrated biochemical residence times spanning from minutes to 7 d. An inverted cyanoacrylamide with prolonged residence time in vivo remained bound to BTK for more than 18 h after clearance from the circulation. The inverted cyanoacrylamide strategy was further used to discover fibroblast growth factor receptor (FGFR) kinase inhibitors with residence times of several days, demonstrating the generalizability of the approach. Targeting of noncatalytic cysteines with inverted cyanoacrylamides may serve as a broadly applicable platform that facilitates 'residence time by design', the ability to modulate and improve the duration of target engagement in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Copeland, R.A., Pompliano, D.L. & Meek, T.D. Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discov. 5, 730–739 (2006).
Copeland, R.A. The dynamics of drug-target interactions: drug-target residence time and its impact on efficacy and safety. Expert Opin. Drug Discov. 5, 305–310 (2010).
Lu, H. & Tonge, P.J. Drug-target residence time: critical information for lead optimization. Curr. Opin. Chem. Biol. 14, 467–474 (2010).
Guo, D., Hillger, J.M., IJzerman, A.P. & Heitman, L.H. Drug-target residence time—a case for G protein-coupled receptors. Med. Res. Rev. 34, 856–892 (2014).
Swinney, D.C. et al. A study of the molecular mechanism of binding kinetics and long residence times of human CCR5 receptor small molecule allosteric ligands. Br. J. Pharmacol. 171, 3364–3375 (2014).
Louvel, J. et al. Agonists for the adenosine A1 receptor with tunable residence time. A case for nonribose 4-amino-6-aryl-5-cyano-2-thiopyrimidines. J. Med. Chem. 57, 3213–3222 (2014).
Vilums, M. et al. Structure-kinetic relationships—an overlooked parameter in hit-to-lead optimization: a case of cyclopentylamines as chemokine receptor 2 antagonists. J. Med. Chem. 56, 7706–7714 (2013).
Miller, R.M., Paavilainen, V.O., Krishnan, S., Serafimova, I.M. & Taunton, J. Electrophilic fragment-based design of reversible covalent kinase inhibitors. J. Am. Chem. Soc. 135, 5298–5301 (2013).
Serafimova, I.M. et al. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat. Chem. Biol. 8, 471–476 (2012).
Barf, T. & Kaptein, A. Irreversible protein kinase inhibitors: balancing the benefits and risks. J. Med. Chem. 55, 6243–6262 (2012).
Mah, R., Thomas, J.R. & Shafer, C.M. Drug discovery considerations in the design of covalent inhibitors. Bioorg. Med. Chem. 24, 33–39 (2014).
Kalgutkar, A.S. & Dalvie, D.K. Drug discovery for a new generation of covalent drugs. Expert Opin. Drug Discov. 7, 561–581 (2012).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Leproult, E., Barluenga, S., Moras, D., Wurtz, J.M. & Winssinger, N. Cysteine mapping in conformationally distinct kinase nucleotide binding sites: application to the design of selective covalent inhibitors. J. Med. Chem. 54, 1347–1355 (2011).
Liu, Q. et al. Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol. 20, 146–159 (2013).
Singh, J., Petter, R.C. & Kluge, A.F. Targeted covalent drugs of the kinase family. Curr. Opin. Chem. Biol. 14, 475–480 (2010).
Honigberg, L.A. et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 107, 13075–13080 (2010).
Evans, E.K. et al. Inhibition of BTK with CC-292 provides early pharmacodynamics assessment of activity in mice and humans. J. Pharmacol. Exp. Ther. 346, 219–228 (2013).
Rankin, A.L. et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J. Immunol. 191, 4540–4550 (2013).
Benson, M.J. et al. Modeling the clinical phenotype of BTK inhibition in the mature murine immune system. J. Immunol. 193, 185–197 (2014).
Wu, H. et al. Discovery of a potent, covalent BTK inhibitor for B-cell lymphoma. ACS Chem. Biol. 9, 1086–1091 (2014).
Akinleye, A., Chen, Y., Mukhi, N., Song, Y. & Liu, D. Ibrutinib and novel BTK inhibitors in clinical development. J. Hematol. Oncol. 6, 59 (2013).
Lou, Y., Owens, T.D., Kuglstatter, A., Kondru, R.K. & Goldstein, D.M. Bruton's tyrosine kinase inhibitors: approaches to potent and selective inhibition, preclinical and clinical evaluation for inflammatory diseases and B cell malignancies. J. Med. Chem. 55, 4539–4550 (2012).
Byrd, J.C. et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 369, 32–42 (2013).
Wang, M.L. et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 369, 507–516 (2013).
Di Paolo, J.A. et al. Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat. Chem. Biol. 7, 41–50 (2011).
Xu, D. et al. RN486, a selective Bruton's tyrosine kinase inhibitor, abrogates immune hypersensitivity responses and arthritis in rodents. J. Pharmacol. Exp. Ther. 341, 90–103 (2012).
Lanning, B.R. et al. A road map to evaluate the proteome-wide selectivity of covalent kinase inhibitors. Nat. Chem. Biol. 10, 760–767 (2014).
Kuglstatter, A. et al. Insights into the conformational flexibility of Bruton's tyrosine kinase from multiple ligand complex structures. Protein Sci. 20, 428–436 (2011).
Marcotte, D.J. et al. Structures of human Bruton's tyrosine kinase in active and inactive conformations suggest a mechanism of activation for TEC family kinases. Protein Sci. 19, 429–439 (2010).
Evans, D.A., Ennis, M.D., Le, T., Mandel, N. & Mandel, G. Asymmetric acylation reactions of chiral imide enolates. The first direct approach to the construction of chiral β-dicarbonyl synthons. J. Am. Chem. Soc. 106, 1154–1156 (1984).
Lebakken, C.S. et al. Development and validation of a broad-coverage, TR-FRET-based kinase binding assay platform. J. Biomol. Screen. 14, 924–935 (2009).
Motulsky, H.J. & Mahan, L.C. The kinetics of competitive radioligand binding predicted by the law of mass action. Mol. Pharmacol. 25, 1–9 (1984).
Copeland, R.A. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists, 2nd edn. (John Wiley & Sons, 2013).
Hantschel, O. et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc. Natl. Acad. Sci. USA 104, 13283–13288 (2007).
Eglen, R.M. et al. The use of AlphaScreen technology in HTS: current status. Curr. Chem. Genomics 1, 2–10 (2008).
Zhou, W. et al. A structure-guided approach to creating covalent FGFR inhibitors. Chem. Biol. 17, 285–295 (2010).
Guagnano, V. et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Discov. 2, 1118–1133 (2012).
Selinsky, B.S., Gupta, K., Sharkey, C.T. & Loll, P.J. Structural analysis of NSAID binding by prostaglandin H2 synthase: time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry 40, 5172–5180 (2001).
Swinney, D.C. Can binding kinetics translate to a clinically differentiated drug? From theory to practice. Lett. Drug Des. Discov. 3, 569–574 (2006).
Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 5, 160–170 (2006).
Vauquelin, G., Bostoen, S., Vanderheyden, P. & Seeman, P. Clozapine, atypical antipsychotics, and the benefits of fast-off D2 dopamine receptor antagonism. Naunyn Schmiedebergs Arch. Pharmacol. 385, 337–372 (2012).
Kapur, S. & Seeman, P. Antipyschotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J. Psychiatry Neurosci. 25, 161–166 (2000).
Dubovsky, J.A. et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 122, 2539–2549 (2013).
Nakayama, S. et al. A zone classification system for risk assessment of idiosyncratic drug toxicity using daily dose and covalent binding. Drug Metab. Dispos. 37, 1970–1977 (2009).
Takakusa, H. et al. Covalent binding and tissue distribution/retention assessment of drugs associated with idiosyncratic drug toxicity. Drug Metab. Dispos. 36, 1770–1779 (2008).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
This work was supported by the US National Institutes of Health (NIH) (grants GM071434 to J.T. and F32GM087052 to J.M.M.), the University of California San Francisco (UCSF) Stephen & Nancy Grand Multiple Myeloma Translational Initiative (to J.T.), the Academy of Finland (to V.O.P.) and the Sigrid Juselius Foundation (to V.O.P.). We acknowledge the UCSF Mass Spectrometry Facility (supported by NIH grant P41RR001614).
Author information
Authors and Affiliations
Contributions
J.M.M., V.O.P. and J.T. designed experiments involving compounds 1–3; J.M.M. and V.O.P. performed experiments and analyzed data for those compounds. J.M.B., A.B., D.T., V.T.P., S.R., P.A.N., D.E.K., M.E.G., J.O.F., T.D.O., E.V., K.A.B., R.J.H. and D.M.G. designed and managed experiments involving compounds 4–46; J.M.B., A.B., D.T., V.T.P., S.R., D.F., J.S., V.P., T.T., X.L. and D.G.L. performed experiments and analyzed data for those compounds. J.M.B. and J.T. wrote the manuscript with feedback from other authors, and all authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.T., J.M.M. and V.O.P. have filed patent applications on cyanoacrylamide kinase inhibitors (licensed to Principia Biopharma, of which J.T. is a cofounder). J.M.B., A.B., D.T., V.T.P., D.F., J.S., V.P., T.T., X.L., D.G.L., P.A.N., D.E.K., M.E.G., J.O.F., T.D.O., E.V., K.A.B., R.J.H. and D.M.G. are members of Principia Biopharma, which is interested in developing BTK inhibitors for therapeutic applications.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–25, Supplementary Tables 1–6 and Supplementary Note. (PDF 4153 kb)
Rights and permissions
About this article
Cite this article
Bradshaw, J., McFarland, J., Paavilainen, V. et al. Prolonged and tunable residence time using reversible covalent kinase inhibitors. Nat Chem Biol 11, 525–531 (2015). https://doi.org/10.1038/nchembio.1817
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1817
This article is cited by
-
Preclinical evaluation of the SARS-CoV-2 Mpro inhibitor RAY1216 shows improved pharmacokinetics compared with nirmatrelvir
Nature Microbiology (2024)
-
Recent progress in ITP treatment
International Journal of Hematology (2023)
-
Identification of natural potent inhibitors against Mycobacterium tuberculosis isocitrate lyase: an in silico study
Molecular Diversity (2023)
-
An update on the discovery and development of reversible covalent inhibitors
Medicinal Chemistry Research (2023)
-
Advances in covalent drug discovery
Nature Reviews Drug Discovery (2022)