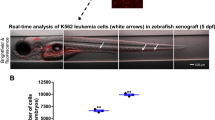
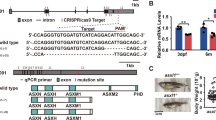
Abstract
It has been proposed that inhibitors of an oncogene's effects on multipotent hematopoietic progenitor cell differentiation may change the properties of the leukemic stem cells and complement the clinical use of cytotoxic drugs. Using zebrafish, we developed a robust in vivo hematopoietic differentiation assay that reflects the activity of the oncogene AML1-ETO. Screening for modifiers of AML1-ETO–mediated hematopoietic dysregulation uncovered unexpected roles of COX-2– and β-catenin–dependent pathways in AML1-ETO function. This approach may open doors for developing therapeutics targeting oncogene function within leukemic stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Redaelli, A., Botteman, M.F., Stephens, J.M., Brandt, S. & Pashos, C.L. Economic burden of acute myeloid leukemia: a literature review. Cancer Treat. Rev. 30, 237–247 (2004).
Guan, Y., Gerhard, B. & Hogge, D.E. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood 101, 3142–3149 (2003).
Terpstra, W. et al. Fluorouracil selectively spares acute myeloid leukemia cells with long-term growth abilities in immunodeficient mice and in culture. Blood 88, 1944–1950 (1996).
Wang, J.C. & Dick, J.E. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005).
Tenen, D.G. Disruption of differentiation in human cancer: AML shows the way. Nat. Rev. Cancer 3, 89–101 (2003).
Choi, Y., Elagib, K.E., Delehanty, L.L. & Goldfarb, A.N. Erythroid inhibition by the leukemic fusion AML1-ETO is associated with impaired acetylation of the major erythroid transcription factor GATA-1. Cancer Res. 66, 2990–2996 (2006).
Schwieger, M. et al. AML1-ETO inhibits maturation of multiple lymphohematopoietic lineages and induces myeloblast transformation in synergy with ICSBP deficiency. J. Exp. Med. 196, 1227–1240 (2002).
Fenske, T.S. et al. Stem cell expression of the AML1/ETO fusion protein induces a myeloproliferative disorder in mice. Proc. Natl. Acad. Sci. USA 101, 15184–15189 (2004).
de Guzman, C.G. et al. Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation. Mol. Cell. Biol. 22, 5506–5517 (2002).
Zon, L.I. & Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 4, 35–44 (2005).
Galloway, J.L., Wingert, R.A., Thisse, C., Thisse, B. & Zon, L.I. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev. Cell 8, 109–116 (2005).
Rhodes, J. et al. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev. Cell 8, 97–108 (2005).
Yeh, J.R. et al. AML1-ETO reprograms hematopoietic cell fate by downregulating scl expression. Development 135, 401–410 (2008).
Yamasaki, H. et al. High degree of myeloid differentiation and granulocytosis is associated with t(8;21) smoldering leukemia. Leukemia 9, 1147–1153 (1995).
Nakamura, H. et al. Morphological subtyping of acute myeloid leukemia with maturation (AML-M2): homogeneous pink-colored cytoplasm of mature neutrophils is most characteristic of AML-M2 with t(8;21). Leukemia 11, 651–655 (1997).
Gottlicher, M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann. Hematol. 83 (Suppl. 1), S91–S92 (2004).
Gottlicher, M. et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20, 6969–6978 (2001).
Liu, S. et al. Targeting AML1/ETO-histone deacetylase repressor complex: a novel mechanism for valproic acid-mediated gene expression and cellular differentiation in AML1/ETO-positive acute myeloid leukemia cells. J. Pharmacol. Exp. Ther. 321, 953–960 (2007).
Bug, G. et al. Effect of histone deacetylase inhibitor valproic acid on progenitor cells of acute myeloid leukemia. Haematologica 92, 542–545 (2007).
Grosser, T., Yusuff, S., Cheskis, E., Pack, M.A. & FitzGerald, G.A. Developmental expression of functional cyclooxygenases in zebrafish. Proc. Natl. Acad. Sci. USA 99, 8418–8423 (2002).
North, T.E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007).
Ishikawa, T.O., Griffin, K.J., Banerjee, U. & Herschman, H.R. The zebrafish genome contains two inducible, functional cyclooxygenase-2 genes. Biochem. Biophys. Res. Commun. 352, 181–187 (2007).
Williams, C.S., Mann, M. & DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18, 7908–7916 (1999).
Cha, Y.I. et al. Cyclooxygenase-1-derived PGE2 promotes cell motility via the G-protein-coupled EP4 receptor during vertebrate gastrulation. Genes Dev. 20, 77–86 (2006).
Cha, Y.I., Kim, S.H., Solnica-Krezel, L. & Dubois, R.N. Cyclooxygenase-1 signaling is required for vascular tube formation during development. Dev. Biol. 282, 274–283 (2005).
Sonoshita, M. et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat. Med. 7, 1048–1051 (2001).
Oshima, M. et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87, 803–809 (1996).
Cha, Y.I. & DuBois, R.N. NSAIDs and cancer prevention: targets downstream of COX-2. Annu. Rev. Med. 58, 239–252 (2007).
Wang, D. et al. Prostaglandin E2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell 6, 285–295 (2004).
Castellone, M.D., Teramoto, H., Williams, B.O., Druey, K.M. & Gutkind, J.S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science 310, 1504–1510 (2005).
Shao, J., Jung, C., Liu, C. & Sheng, H. Prostaglandin E2 stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J. Biol. Chem. 280, 26565–26572 (2005).
Lyman Gingerich, J., Westfall, T.A., Slusarski, D.C. & Pelegri, F. hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev. Biol. 286, 427–439 (2005).
Meijer, L. et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 10, 1255–1266 (2003).
van de Water, S. et al. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development 128, 3877–3888 (2001).
Stachel, S.E., Grunwald, D.J. & Myers, P.Z. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development 117, 1261–1274 (1993).
Fujino, H., West, K.A. & Regan, J.W. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 277, 2614–2619 (2002).
Hino, S., Tanji, C., Nakayama, K.I. & Kikuchi, A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 25, 9063–9072 (2005).
Trowbridge, J.J., Xenocostas, A., Moon, R.T. & Bhatia, M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat. Med. 12, 89–98 (2006).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Cobas, M. et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199, 221–229 (2004).
Dinchuk, J.E. et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378, 406–409 (1995).
Langenbach, R. et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83, 483–492 (1995).
Morham, S.G. et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83, 473–482 (1995).
Patton, E.E. & Zon, L.I. Taking human cancer genes to the fish: a transgenic model of melanoma in zebrafish. Zebrafish 1, 363–368 (2005).
Nasevicius, A. & Ekker, S.C. Effective targeted gene 'knockdown' in zebrafish. Nat. Genet. 26, 216–220 (2000).
Schulte-Merker, S., Ho, R.K., Herrmann, B.G. & Nusslein-Volhard, C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116, 1021–1032 (1992).
Thompson, M.A. et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 197, 248–269 (1998).
Bennett, C.M. et al. Myelopoiesis in the zebrafish, Danio rerio. Blood 98, 643–651 (2001).
Kalev-Zylinska, M.L. et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1–CBF2T1 transgene advances a model for studies of leukemogenesis. Development 129, 2015–2030 (2002).
van de Wetering, M. et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
Acknowledgements
We thank E.R. Plovie and M.N. Rivera (Massachusetts General Hospital) and H. Clevers (Hubrecht Institute) for providing reagents, and C.L. Tsai, C. Sachidanandan and the members of the Developmental Biology Laboratory for helpful discussion. J.-R.J.Y. is supported by a Career Development Award (AG031300) from the National Institute of Aging. The authors received financial support from the National Cancer Institute (CA118498 to R.T.P.), the Mattina Proctor Foundation (to D.A.S) and the Claflin Distinguished Scholar Award (to J.-R.J.Y.).
Author information
Authors and Affiliations
Contributions
J.-R.J.Y designed and performed experiments, interpreted data and wrote the manuscript; K.M.M. designed and performed experiments and interpreted data; K.E.E. and A.N.G. provided critical reagents and advice; D.A.S. provided critical advice and edited the manuscript; R.T.P. designed experiments, interpreted data and edited the manuscript.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Table 1 (PDF 538 kb)
Rights and permissions
About this article
Cite this article
Yeh, JR., Munson, K., Elagib, K. et al. Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat Chem Biol 5, 236–243 (2009). https://doi.org/10.1038/nchembio.147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.147
This article is cited by
-
Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials
Nature Reviews Drug Discovery (2021)
-
Zebrafish and Medaka: new model organisms for modern biomedical research
Journal of Biomedical Science (2016)