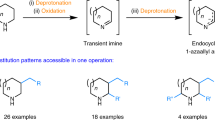
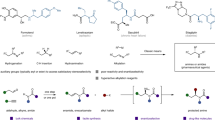
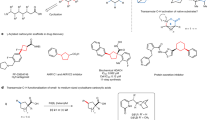
Abstract
Cyclic amines are ubiquitous core structures of bioactive natural products and pharmaceutical drugs. Although the site-selective abstraction of C–H bonds is an attractive strategy for preparing valuable functionalized amines from their readily available parent heterocycles, this approach has largely been limited to substrates that require protection of the amine nitrogen atom. In addition, most methods rely on transition metals and are incompatible with the presence of amine N–H bonds. Here we introduce a protecting-group-free approach for the α-functionalization of cyclic secondary amines. An operationally simple one-pot procedure generates products via a process that involves intermolecular hydride transfer to generate an imine intermediate that is subsequently captured by a nucleophile, such as an alkyl or aryl lithium compound. Reactions are regioselective and stereospecific and enable the rapid preparation of bioactive amines, as exemplified by the facile synthesis of anabasine and (–)-solenopsin A.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Vo, C.-V. T. & Bode, J. W. Synthesis of saturated N-heterocycles. J. Org. Chem. 79, 2809–2815 (2014).
Campos, K. R. Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 36, 1069–1084 (2007).
Mitchell, E. A., Peschiulli, A., Lefevre, N., Meerpoel, L. & Maes, B. U. W. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 18, 10092–10142 (2012).
Beak, P. & Lee, W.-K. α-lithioamine synthetic equivalents from dipole-stabilized carbanions: the t-Boc group as an activator for α′-lithiation of carbamates. Tetrahedron Lett. 30, 1197–1200 (1989).
Beak, P., Kerrick, S. T., Wu, S. & Chu, J. Complex induced proximity effects: enantioselective syntheses based on asymmetric deprotonations of Noc-pyrrolidines. J. Am. Chem. Soc. 116, 3231–3239 (1994).
McGrath, M. J. & O'Brien, P. Catalytic asymmetric deprotonation using a ligand exchange approach. J. Am. Chem. Soc. 127, 16378–16379 (2005).
Seel, S. et al. Highly diastereoselective arylations of substituted piperidines. J. Am. Chem. Soc. 133, 4774–4777 (2011).
Beng, T. K., Woo, J. S. & Gawley, R. E. Synthetic applications and inversion dynamics of configurationally stable 2-lithio-2-arylpyrrolidines and -piperidines. J. Am. Chem. Soc. 134, 14764–14771 (2012).
Cordier, C. J., Lundgren, R. J. & Fu, G. C. Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α-alkylations of Noc-pyrrolidine. J. Am. Chem. Soc. 135, 10946–10949 (2013).
Li, X. & Coldham, I. Synthesis of 1,1-disubstituted tetrahydroisoquinolines by lithiation and substitution, with in situ IR spectroscopy and configurational stability studies. J. Am. Chem. Soc. 136, 5551–5554 (2014).
Campos, K. R., Klapars, A., Waldman, J. H., Dormer, P. G. & Chen, C.-Y. Enantioselective, palladium-catalyzed α-arylation of Noc-pyrrolidine. J. Am. Chem. Soc. 128, 3538–3539 (2006).
Pastine, S. J., Gribkov, D. V. & Sames, D. sp3 C–H bond arylation directed by amidine protecting group: α-arylation of pyrrolidines and piperidines. J. Am. Chem. Soc. 128, 14220–14221 (2006).
Spangler, J. E., Kobayashi, Y., Verma, P., Wang, D.-H. & Yu, J.-Q. α-arylation of saturated azacycles and N-methylamines via palladium(II)-catalyzed C(sp3)–H coupling. J. Am. Chem. Soc. 137, 11876–11879 (2015).
Jain, P., Verma, P., Xia, G. & Yu, J.-Q. Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides. Nat. Chem. 9, 140–144 (2017).
Shono, T., Matsumura, Y. & Tsubata, K. Electroorganic chemistry. 46. A new carbon–carbon bond forming reaction at the α-position of amines utilizing anodic oxidation as a key step. J. Am. Chem. Soc. 103, 1172–1176 (1981).
Li, Z. P. & Li, C. J. CuBr-catalyzed efficient alkynylation of sp3 C–H bonds adjacent to a nitrogen atom. J. Am. Chem. Soc. 126, 11810–11811 (2004).
Girard, S. A., Knauber, T. & Li, C.-J. The cross-dehydrogenative coupling of C(sp3)–H bonds: a versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 53, 74–100 (2014).
McNally, A., Prier, C. K. & MacMillan, D. W. C. Discovery of an α-amino C–H arylation reaction using the strategy of accelerated serendipity. Science 334, 1114–1117 (2011).
Shaw, M. H., Shurtleff, V. W., Terrett, J. A., Cuthbertson, J. D. & MacMillan, D. W. C. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science 352, 1304–1308 (2016).
Beatty, J. W. & Stephenson, C. R. J. Amine functionalization via oxidative photoredox catalysis: methodology development and complex molecule synthesis. Acc. Chem. Res. 48, 1474–1484 (2015).
Yoshikai, N., Mieczkowski, A., Matsumoto, A., Ilies, L. & Nakamura, E. Iron-catalyzed C–C bond formation at α-position of aliphatic amines via C–H bond activation through 1,5-hydrogen transfer. J. Am. Chem. Soc. 132, 5568–5569 (2010).
Haibach, M. C. & Seidel, D. C–H bond functionalization through intramolecular hydride transfer. Angew. Chem. Int. Ed. 53, 5010–5036 (2014).
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Millet, A., Larini, P., Clot, E. & Baudoin, O. Ligand-controlled β-selective C(sp3)–H arylation of Noc-piperidines. Chem. Sci. 4, 2241–2247 (2013).
Topczewski, J. J., Cabrera, P. J., Saper, N. I. & Sanford, M. S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016).
Payne, P. R., Garcia, P., Eisenberger, P., Yim, J. C. H. & Schafer, L. L. Tantalum catalyzed hydroaminoalkylation for the synthesis of α- and β-substituted N-heterocycles. Org. Lett. 15, 2182–2185 (2013).
Zhang, C., De, C. K., Mal, R. & Seidel, D. α-amination of nitrogen heterocycles: ring-fused aminals. J. Am. Chem. Soc. 130, 416–417 (2008).
Seidel, D. The azomethine ylide route to amine C–H functionalization: redox versions of classic reactions and a pathway to new transformations. Acc. Chem. Res. 48, 317–328 (2015).
Majewski, M. & Gleave, D. M. Reduction with lithium dialkylamides. J. Organomet. Chem. 470, 1–16 (1994).
Scully, F. E. Regioselective 2-alkylation and 2-arylation of piperidine and pyrrolidine via organolithiation of cyclic imines. J. Org. Chem. 45, 1515–1517 (1980).
Wittig, G. & Hesse, A. Zur Reaktionsweise N-metallierter acyclischer und cyclischer sekundärer amine. Liebigs Ann. Chem. 746, 149–173 (1971).
Yujiro, N., Keiichiro, O., Yoshito, T. & Shuji, T. One-step synthesis and structural confirmation of 1-pyrroline trimer. Chem. Lett. 6, 693–696 (1977).
MacConnell, J. G., Blum, M. S. & Fales, H. M. Alkaloid from fire ant venom: identification and synthesis. Science 168, 840–841 (1970).
Crooks, P. A. in Analytical Determination of Nicotine and Related Compounds and their Metabolites (eds Gorrod, J. W. & Jacob, P. III ) 69–147 (Elsevier, 1999).
Coe, J. W. et al. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J. Med. Chem. 48, 3474–3477 (2005).
Colpaert, F. C. Discovering risperidone: the LSD model of psychopathology. Nat. Rev. Drug Discov. 2, 315–320 (2003).
Yamataka, H., Kawafuji, Y., Nagareda, K., Miyano, N. & Hanafusa, T. Electron transfer in the additions of organolithium reagents to benzophenone and benzaldehyde. J. Org. Chem. 54, 4706–4708 (1989).
Yamataka, H., Miyano, N. & Hanafusa, T. Comparative mechanistic study of the reactions of benzophenone with N-butylmagnesium bromide and N-butyllithium. J. Org. Chem. 56, 2573–2575 (1991).
Acknowledgements
Financial support from the NIH–NIGMS (R01GM101389) is gratefully acknowledged. We thank T. Emge (Rutgers University) for the crystallographic analysis.
Author information
Authors and Affiliations
Contributions
W.C. and L.M. developed the amine α-functionalization and contributed equally to this work. A.P. further developed the reaction and expanded the scope. D.S. conceived and supervised the project and wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7926 kb)
Supplementary information
Crystallographic data for compound ±45. (CIF 148 kb)
Rights and permissions
About this article
Cite this article
Chen, W., Ma, L., Paul, A. et al. Direct α-C–H bond functionalization of unprotected cyclic amines. Nature Chem 10, 165–169 (2018). https://doi.org/10.1038/nchem.2871
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2871
This article is cited by
-
Synthesis of dienes from pyrrolidines using skeletal modification
Nature Communications (2023)
-
Site- and enantioselective cross-coupling of saturated N-heterocycles with carboxylic acids by cooperative Ni/photoredox catalysis
Nature Communications (2023)
-
Isolable iminium ions as a platform for N-(hetero)aryl piperidine synthesis
Nature Synthesis (2023)
-
Organogel delivery vehicles for the stabilization of organolithium reagents
Nature Chemistry (2023)
-
Enantioselective oxidation of unactivated C–H bonds in cyclic amines by iterative docking-guided mutagenesis of P450BM3 (CYP102A1)
Nature Synthesis (2022)