Abstract
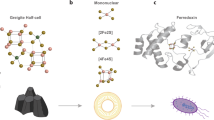
Iron–sulfur clusters are ancient cofactors that play a fundamental role in metabolism and may have impacted the prebiotic chemistry that led to life. However, it is unclear whether iron–sulfur clusters could have been synthesized on prebiotic Earth. Dissolved iron on early Earth was predominantly in the reduced ferrous state, but ferrous ions alone cannot form polynuclear iron–sulfur clusters. Similarly, free sulfide may not have been readily available. Here we show that UV light drives the synthesis of [2Fe–2S] and [4Fe–4S] clusters through the photooxidation of ferrous ions and the photolysis of organic thiols. Iron–sulfur clusters coordinate to and are stabilized by a wide range of cysteine-containing peptides and the assembly of iron–sulfur cluster-peptide complexes can take place within model protocells in a process that parallels extant pathways. Our experiments suggest that iron–sulfur clusters may have formed easily on early Earth, facilitating the emergence of an iron–sulfur-cluster-dependent metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eck, R. V. & Dayhoff, M. O. Evolution of the structure of ferredoxin based on living relics of primitive amino acid sequences. Science 152, 363–366 (1966).
Goldford, J. E., Hartman, H., Smith, T. F. & Segrè, D. Remnants of an ancient metabolism without phosphate. Cell 168, 1126–1134 (2017).
Martin, W. & Russell, M. J. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil. Trans. R. Soc. B 358, 59–85 (2003).
Martin, W. F., Sousa, F. L. & Lane, N. Energy at life's origin. Science 344, 1092–1093 (2014).
Maurel, M. & Leclerc, F. From foundation stones to life: concepts and results. Elements 12, 407–412 (2016).
Scintilla, S. et al. Duplications of an iron–sulphur tripeptide leads to the formation of a protoferredoxin. Chem. Commun. 52, 13456–13459 (2016).
Anbar, A. D. Elements and evolution. Science 322, 1481–1483 (2008).
Osterberg, R. Origins of metal ions in biology. Nature 249, 382–383 (1974).
Blöchl, E., Keller, M., Wachtershäuser, G. & Stetter, K. O. Reactions depending on iron sulfide and linking geochemistry with biochemistry. Proc. Natl Acad. Sci. USA 89, 8117–8120 (1992).
Cody, G. D. et al. Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science 289, 1337–1340 (2000).
Ritson, D. & Sutherland, J. D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 4, 895–899 (2012).
Patel, B. H., Percivalle, C., Ritson, D. J., Duffy, C. D. & Sutherland, J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015).
Qi, W. et al. Glutathione complexed Fe–S centers. J. Am. Chem. Soc. 134, 10745–10748 (2012).
Fiser, B. et al. Glutathione as a prebiotic answer to α-peptide based life. J. Phys. Chem. B 119, 3940–3947 (2015).
Pandelia, M. E., Lanz, N. D., Booker, S. J. & Krebs, C. Mössbauer spectroscopy of Fe/S proteins. Biochim. Biophys. Acta 1853, 1395–1405 (2015).
Duin, E. C. et al. [2Fe-2S] to [4Fe-4S] cluster conversion in Escherichia coli biotin synthase. Biochemistry 36, 11811–11820 (1997).
Pasek, M. A., Kee, T. P., Bryant, D. E., Pavlov, A. A. & Lunine, J. I. Production of potentially prebiotic condensed phosphates by phosphorus redox chemistry. Angew. Chem. Int. Ed. 47, 7918–7920 (2008).
Burcar, B. et al. Darwin's warm little pond: a one-pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea-based solvent. Angew. Chem. Int. Ed. 55, 13249–13253 (2016).
Venkateswara Rao, P. & Holm, R. H . Synthetic analogues of the active sites of iron–sulfur proteins. Chem. Rev. 104, 527–559 (2004).
Ranjan, S. & Sasselov, D. D. Influence of the UV environment on the synthesis of prebiotic molecules. Astrobiology 16, 68–88 (2016).
Braterman, P. S., Cairns-Smith, A. G., Sloper, R. W., Truscott, T. G. & Craw, M. Photo-oxidation of iron(II) in water between pH 7.5 and 4.0. J. Chem. Soc. Dalton Trans. 7, 1441–1445 (1984).
Lill, R. Function and biogenesis of iron–sulphur proteins. Nature 460, 831–838 (2009).
Qi, W. & Cowan, J. A. Structural, mechanistic and coordination chemistry of relevance to the biosynthesis of iron–sulfur and related iron cofactors. Coord. Chem. Rev. 255, 688–699 (2011).
Mihara, H. & Esaki, N. Bacterial cysteine desulfurases: their function and mechanisms. Appl. Microbiol. Biotechnol. 60, 12–23 (2003).
Liu, Y. et al. A [3Fe–4S] cluster is required for tRNA thiolation in archaea and eukaryotes. Proc. Natl Acad. Sci. USA 113, 12703–12708 (2016).
Gil, R., Silva, F. J., Pereto, J. & Moya, A. Determination of the core of a minimal bacterial gene set. Microbiol. Mol. Biol. Rev. 68, 518–537 (2004).
Rapf, R. J. & Vaida, V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys. Chem. Chem. Phys. 18, 20067–20084 (2016).
Kim, J. H., Bothe, J. R., Alderson, T. R. & Markley, J. L. Tangled web of interactions among proteins involved in iron–sulfur cluster assembly as unraveled by NMR, SAXS, chemical crosslinking, and functional studies. Biochim. Biophys. Acta 1853, 1416–1428 (2015).
Lide, D. R. (ed.) CRC Handbook of Chemistry and Physics 84th edn (CRC, 2003).
Yang, C. S. & Huennekens, F. M. Iron–mercaptoethanol–inorganic sulfide complex. Possible model for chromophore of nonheme iron proteins. Biochemistry 9, 2127–2133 (1970).
Sugiura, Y. & Tanaka, H. Iron–sulfide chelates of some sulfur-containing peptides as model complex of non-heme iron proteins. Biochem. Biophys. Res. Commun. 46, 335–340 (1972).
Mansy, S. S. & Szostak, J. W. Reconstructing the emergence of cellular life through the synthesis of model protocells. Cold Spring Harb. Symp. Quant. Biol. 74, 47–54 (2009).
Stano, P. & Luisi, P. L. Achievements and open questions in the self-reproduction of vesicles and synthetic minimal cells. Chem. Commun. 46, 3639–3653 (2010).
Monnard, P., Apel, C. L., Kanavarioti, A. & Deamer, D. W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: implications for a prebiotic aqueous medium. Astrobiology 2, 139–152 (2002).
Adamala, K. & Szostak, J. W. Nonenzymatic template-directed RNA synthesis inside model protocells. Science 342, 1098–1100 (2013).
Belmonte, L. & Mansy, S. S. Metal catalysts and the origin of life. Elements 12, 413–418 (2016).
Hsiao, C. et al. RNA with iron(II) as a cofactor catalyses electron transfer. Nat. Chem. 5, 525–528 (2013).
Acknowledgements
The authors acknowledge the Simons Foundation (290360 to D.D.S., 290363 to J.W.S., 290362 to J.D.S., 290358 to S.S.M.), the Armenise-Harvard Foundation (to S.S.M.), COST action CM1304 (to C.B., J.D.S. and S.S.M.) and the University of Hull (to D.J.E. and S.Sh.) for funding. The authors thank L. Belmonte, C. Caumes, E. Izgu, E. Godino, N. Kamat, A. Mariani, T. Olsen, D. Rossetto, Z. Todd, O.D. Toparlak, A. Trifonov and M. Tsanakopoulou for discussions.
Author information
Authors and Affiliations
Contributions
C.B., S.Sc., L.J., J.W.S., D.D.S., J.D.S. and S.S.M. designed the experiments. Photochemical studies, peptide synthesis and cluster stability were performed by C.B. and L.V. Mössbauer spectra were recorded and analysed by S.Sh. and D.J.E. The manuscript was written by C.B. and S.S.M. and edited by C.B., S.Sc., D.J.E., J.W.S., D.D.S., J.D.S. and S.S.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7991 kb)
Rights and permissions
About this article
Cite this article
Bonfio, C., Valer, L., Scintilla, S. et al. UV-light-driven prebiotic synthesis of iron–sulfur clusters. Nature Chem 9, 1229–1234 (2017). https://doi.org/10.1038/nchem.2817
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2817
This article is cited by
-
Ferroptosis regulation through Nrf2 and implications for neurodegenerative diseases
Archives of Toxicology (2024)
-
An inorganic mineral-based protocell with prebiotic radiation fitness
Nature Communications (2023)
-
Diverse geochemical conditions for prebiotic chemistry in shallow-sea alkaline hydrothermal vents
Nature Geoscience (2022)
-
Spontaneous assembly of redox-active iron-sulfur clusters at low concentrations of cysteine
Nature Communications (2021)
-
How the first life on Earth survived its biggest threat — water
Nature (2020)