Abstract
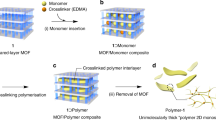
The fabrication of crystalline 2D conjugated polymers with well-defined repeating units and in-built porosity presents a significant challenge to synthetic chemists. Yet they present an appealing target because of their desirable physical and electronic properties. Here we report the preparation of a 2D conjugated aromatic polymer synthesized via C–C coupling reactions between tetrabromopolyaromatic monomers. Pre-arranged monomers in the bulk crystal undergo C–C coupling driven by endogenous solid-state polymerization to produce a crystalline polymer, which can be mechanically exfoliated into micrometre-sized lamellar sheets with a thickness of 1 nm. Isothermal gas-sorption measurements of the bulk material reveal a dominant pore size of ~0.6 nm, which indicates uniform open channels from the eclipsed stacking of the sheets. When employed as an organic anode in an ambient-temperature sodium cell, the material allows a fast charge/discharge of sodium ions, with impressive reversible capacity, rate capability and stability metrics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Sakamoto, J., van Heijst, J., Lukin, O. & Schlüter, A. D. Two-dimensional polymers: just a dream of synthetic chemists? Angew. Chem. Int. Ed. 48, 1030–11069 (2009).
Perepichka, D. F. & Rosei, F. Extending polymer conjugation into the second dimension. Science 323, 216–217 (2009).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Waller, P. J., Gándara, F. & Yaghi, O. M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 48, 3053–3063 (2015).
Baughman, R. H., Eckardt, H. & Kertez, M. Structure–property predictions for new planar forms of carbon: layered phases containing sp2 and sp atoms. J. Chem. Phys. 87, 6687–6699 (1987).
Chen, L., Hernandez, Y., Feng, X. & Müllen, K. From nanographene and graphene nanoribbons to graphene sheets: chemical synthesis. Angew. Chem. Int. Ed. 51, 7640–7654 (2012).
Müllen, K. Evolution of graphene molecules: structural and functional complexity as driving forces behind nanoscience. ACS Nano 8, 6531–6541 (2014).
Simpson, C. D. et al.. Synthesis of a giant 222 carbon graphite sheet. Chem. Eur. J. 8, 1424–1429 (2002).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotech. 2, 687–691 (2007).
Lafferentz, L. et al. Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 4, 215–220 (2012).
Liu, X., Guan, C., Wang, D. & Wan, L. Graphene-like single-layered covalent organic frameworks: synthesis strategies and application prospects. Adv. Mater. 26, 6912–6920 (2014).
Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012).
Wan, S. et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23, 4094–4097 (2011).
Xu, H., Gao, J. & Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Guo, J. et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 4, 2736 (2013).
Groenewolt, M. & Antonietti, M. Synthesis of g-C3N4 nanoparticles in mesoporous silica host matrices. Adv. Mater. 17, 1789–1792 (2005).
Bojdys, M. J., Jeromenok, J., Thomas, A. & Antonietti, M. Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity. Adv. Mater. 22, 2202–2205 (2010).
Bhola, R. et al. A two-dimensional polymer from the anthracene dimer and triptycene motifs. J. Am. Chem. Soc. 135, 14134–14141 (2013).
Kissel, P. et al. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 6, 774–778 (2014).
Kory, M. J. et al. Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction. Nat. Chem. 6, 779–784 (2014).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nat. Chem. 4, 287–291 (2012).
Gritsch, T., Coulman, D., Behm, R. J. & Ertl, G. A scanning tunneling microscopy investigation of the structure of the Pt(110) and Au(110) surfaces. Surf. Sci. 257, 297–306 (1991).
Ben, T. et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 48, 9457–9460 (2009).
Yuan, D., Lu, W., Zhao, D. & Zhou, H. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv. Mater. 23, 3723–3725 (2011).
Stille, J. K. & Mainen, E. L. Thermally stable ladder polyquinoxalines. Macromolecules 1, 36–42 (1968).
Imai, K. et al. Synthesis and properties of thermally stable ladder polymers containing the 1,4-pyrazine ring obtained from polyheterocyclizations of tetramines and tetraketones in poly(phosphoric acid) and m-cresol. Macromolecules 6, 158–162 (1973).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Meng, H., Perepichka, D. F. & Wudl, F. Facile solid-state synthesis of highly conducting poly(ethylenedioxythiophene). Angew. Chem. Int. Ed. 42, 658–661 (2003).
Meng, H. et al. Solid-state synthesis of a conducting polythiophene via an unprecedented heterocyclic coupling reaction. J. Am. Chem. Soc. 125, 15151–15162 (2003).
Newsome, D. A., Sengupta, D., Foroutan, H., Russo, M. F. & van Duin, A. C. T. Oxidation of silicon carbide by O2 and H O2: a ReaxFF reactive molecular dynamics study, part I. J. Phys. Chem. C 116, 1611–1621 (2012).
Weismiller, M. R., van Duin, A. C. T., Lee, J. & Yetter, R. A. ReaxFF reactive force field development and applications for molecular dynamics simulations of ammonia borane dehydrogenation and combustion. J. Phys. Chem. A 114, 5485–5492 (2010).
Islam, M. M., Bryantsev, V. S. & van Duin, A. C. T. ReaxFF reactive force field simulations on the influence of Teflon on electrolyte decomposition during Li/SWCNT anode discharge in lithium–sulfur batteries. J. Electrochem. Soc. 161, 3009–3014 (2014).
Wu, S. M. et al. Molecular junctions based on aromatic coupling. Nat. Nanotech. 3, 569–574 (2008).
Li, H., Powell, D. R., Firman, T. K. & West, R. Structures and photophysical properties of model compounds for arylethynylene disilylene polymers. Macromolecules 31, 1093–1098 (1998).
Wang, C., Batsanov, A., Bryce, M. & Sage, I. Nanoscale aryleneethynylene molecular wires with reversible fluorenone electrochemistry for self-assembly onto metal surfaces. Org. Lett. 6, 2181–2184 (2004).
Larcher, D. & Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Kundu, D., Talaie, E., Duffort, V. & Nazar, L. F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem. Int. Ed. 54, 3431–3448 (2015).
Xu, F. et al. Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 5, 8225 (2015).
Sakaushi, K. et al. Aromatic porous-honeycomb electrodes for a sodium–organic energy storage device. Nat. Commun. 4, 1485 (2013).
Cao, Y. et al. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 12, 3783–3787 (2012).
Ding, J. et al. Carbon nanosheet frameworks derived from peat moss as high performance sodium ion battery anodes. ACS Nano 7, 11004–11015 (2013).
Song, Z. et al. Polymer–graphene nanocomposites as ultrafast charge-and-discharge cathodes for rechargeable lithium batteries. Nano Lett. 12, 2205–2211 (2012).
Wang, D. et al. Layer by layer assembly of sandwiched graphene/SnO2 nanorod/carbon nanostructures with ultrahigh lithium ion storage properties. Energy Environ. Sci. 6, 2900–2906 (2013).
Wang, Z., Luan, D., Madhavi, S., Hu, Y. & Lou, X. W. Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability. Energy Environ. Sci. 5, 5252–5256 (2012).
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008).
Acknowledgements
K.P.L. acknowledges a National Research Foundation (NRF) Competitive Research Programme (CRP) grant NRF2015NRF-CRP002-006 ‘Two-dimensional Covalent Organic Framework: Synthesis and Applications’. K.P. L., S.Y.Q. and the team acknowledge the NRF, Prime Minister's Office, Singapore, under its Medium Sized Centre Programme (CA2DM). S.Y.Q. and X.L. acknowledge the Singapore NRF for funding under the NRF Fellowship (NRFNRFF2013-07). Calculations were performed on the computational cluster at CA2DM, National University of Singapore. The authors thank H. Xu, D. Y. Yu, Y. W. Peng, C. L. Su, S. J. R. Tan, S. M. Poh, X. X. Zhao, D. C. Geng and J. Y. Chen for helpful discussions.
Author information
Authors and Affiliations
Contributions
K.P.L. supervised the project. W.L. and K.P.L. designed and performed the experiments. X.L. performed the theoretical calculations under the supervision of S.Y.Q. Y.B. performed the STM characterization. Y.P.L., I.A. and L.L. helped to exfoliate the sample and conduct the AFM imaging. G.H.N. helped with the crystal files analysis. C.T.N. helped to collect XPS spectra. Z.G.H. and D.Z. helped to perform Ar-sorption measurements. B.L. helped to analyse the experimental results. W.L., X.L., S.Y.Q. and K.P.L. wrote the manuscript with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3818 kb)
Supplementary information
Crystallographic data for 2-TBQP. (CIF 272 kb)
Supplementary information
Crystallographic data for DDQP. (CIF 765 kb)
Rights and permissions
About this article
Cite this article
Liu, W., Luo, X., Bao, Y. et al. A two-dimensional conjugated aromatic polymer via C–C coupling reaction. Nature Chem 9, 563–570 (2017). https://doi.org/10.1038/nchem.2696
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2696
This article is cited by
-
On-surface synthesis of disilabenzene-bridged covalent organic frameworks
Nature Chemistry (2023)
-
Self-assembly and photoinduced fabrication of conductive nanographene wires on boron nitride
Nature Communications (2022)
-
Reconstructed covalent organic frameworks
Nature (2022)
-
Observing polymerization in 2D dynamic covalent polymers
Nature (2022)
-
An aqueous rechargeable zinc-ion battery on basis of an organic pigment
Rare Metals (2022)