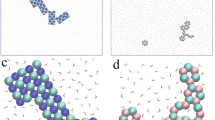
Abstract
Strategies for forming liquid dispersions of nanomaterials typically focus on retarding reaggregation, for example via surface modification, as opposed to promoting the thermodynamically driven dissolution common for molecule-sized species. Here we demonstrate the true dissolution of a wide range of important 2D nanomaterials by forming layered material salts that spontaneously dissolve in polar solvents yielding ionic solutions. The benign dissolution advantageously maintains the morphology of the starting material, is stable against reaggregation and can achieve solutions containing exclusively individualized monolayers. Importantly, the charge on the anionic nanosheet solutes is reversible, enables targeted deposition over large areas via electroplating and can initiate novel self-assembly upon drying. Our findings thus reveal a unique solution-like behaviour for 2D materials that enables their scalable production and controlled manipulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nicolosi, V., Chowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340, 1226419 (2013).
Ferrari, A. C. et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 7, 4598–4810 (2015).
Stankovich, S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007).
Hummelen, J. C. et al. Preparation and characterization of fulleroid and methanofullerene derivatives. J. Org. Chem. 60, 532–538 (1995).
Sperling, R. A. & Parak, W. J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Phil. Trans. R. Soc. A 368, 1333–1383 (2010).
Zobel, M., Neder, R. B. & Kimber, S. A. Universal solvent restructuring induced by colloidal nanoparticles. Science 347, 292–294 (2015).
Boles, M. A., Ling, D., Hyeon, T. & Talapin, D. V. The surface science of nanocrystals. Nat. Mat. 15, 141–153 (2016).
Lotya, M. et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131, 3611–3620 (2009).
Paton, K. R. et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 13, 624–630 (2014).
Shih, C. J. et al. Bi-and trilayer graphene solutions. Nat. Nanotech. 6, 439–445 (2011).
Coleman, J. N. et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331, 568–571 (2011).
Huo, C., Yan, Z., Song, X. & Zeng, H. 2D materials via liquid exfoliation: a review on fabrication and applications. Sci. Bull. 60, 1994–2008 (2015).
Ma, R. & Sasaki, T. Two-dimensional oxide and hydroxide nanosheets: controllable high-quality exfoliation, molecular assembly, and exploration of functionality. Acc. Chem. Res. 48, 136–143 (2015).
Stöter, M., Rosenfeldt, S. & Breu, J. Tunable exfoliation of synthetic clays. Annu. Rev. Mater. Res. 45, 129–151 (2015).
Batista, C. A. S., Larson, R. G. & Kotov, N. A. Nonadditivity of nanoparticle interactions. Science 350, 6257 (2015).
Howard, C. A., Thompson, H., Wasse, J. C. & Skipper, N. T. Formation of giant solvation shells around fulleride anions in liquid ammonia. J. Am. Chem. Soc. 126, 13228–13229 (2004).
Mezger, M. et al. Molecular layering of fluorinated ionic liquids at a charged sapphire (0001) surface. Science 322, 424–428 (2008).
Burgess, J. Ions in Solution: Basic Principles of Chemical Interactions 2nd edn (Horwood, 1999).
Joensen, P., Frindt, R. F. & Morrison, S. R. Single-layer MoS2 . Mater. Res. Bull. 21, 457–461 (1986).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2 . Nano Lett. 11, 5111–5116 (2011).
Lu, X. et al. Preparation of MoO3 QDs through combining intercalation and thermal exfoliation. J. Mat. Chem. C 4, 6720 (2016).
Schaak, R. E. & Mallouk, T. E. Prying apart ruddlesden-popper phases: exfoliation into sheets and nanotubes for assembly of perovskite thin films. Chem. Mater. 12, 3427–3434 (2000).
Sasaki, T., Watanabe, M., Hashizume, H., Yamada, H. & Nakazawa, H. Macromolecule-like aspects for a colloidal suspension of an exfoliated titanate. Pairwise association of nanosheets and dynamic reassembling process initiated from it. J. Am. Chem. Soc. 118, 8329–8335 (1996).
Sposito, G. et al. Surface geochemistry of the clay minerals. Proc. Natl Acad. Sci. USA 96, 3358–3364 (1999).
Dresselhaus, M. S. & Dresselhaus, G. Intercalation compounds of graphite. Adv. Phys. 51, 1–186 (2002).
Fan, X. et al. Controlled exfoliation of MoS2 crystals into trilayer nanosheets. J. Am. Chem. Soc. 138, 5143–5149 (2016).
Ding, Z., Viculis, L., Nakawatase, J. & Kaner, R. B. Intercalation and solution processing of bismuth telluride and bismuth selenide. Adv. Mater. 13, 797–800 (2001).
Zeng, Z. et al. Single layer semiconducting nanosheets: high-yield preparation and device fabrication. Angew. Chem. Int. Ed. 50, 11093–11097 (2011).
Vallés, C. et al. Solutions of negatively charged graphene sheets and ribbons. J. Am. Chem. Soc. 130, 15802–15804 (2008).
Milner, E. M. et al. Structure and morphology of charged graphene platelets in solution by small-angle neutron scattering. J. Am. Chem. Soc. 134, 8302–8305 (2012).
Huang, K. et al. Single layer nano graphene platelets derived from graphite nanofibres. Nanoscale 8, 8810 (2016).
Pan, Z.-H. et al. Electronic structure of superconducting KC8 and nonsuperconducting LiC6 graphite intercalation compounds: evidence for a graphene-sheet-driven superconducting state. Phys. Rev. Lett. 106, 187002 (2011).
Somoano, R. B., Hadek, V. & Rembaum, A. Alkali metal intercalates of molybdenum disulfide. J. Chem. Phys. 58, 697–701 (1973).
Picco, L. M. et al. Breaking the speed limit with atomic force microscopy. Nanotechnology 18, 044030 (2006).
Zeng, Z., Tan, C., Huang, X., Bao, S. & Zhang, H. Growth of noble metal nanoparticles on single-layer TiS2 and TaS2 nanosheets for hydrogen evolution reaction. Energy Environ. Sci. 7, 797 (2014).
Lui, C. H., Liu, L., Mak, K. F., Flynn, G. W. & Heinz, T. F. Ultraflat graphene. Nature 462, 339–341 (2009).
Tonndorf, P. et al. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2 . Opt. Express 21, 4908–4916 (2013).
Yoon, G. et al. Factors affecting the exfoliation of graphite intercalation compounds for graphene synthesis. Chem. Mater. 27, 2067–2073 (2015).
Payton, O. D. et al. Experimental observation of contact mode cantilever dynamics with nanosecond resolution. Rev. Sci. Instrum. 82, 043704 (2011).
Kodera, N., Yamamoto, D., Ishikawa, R. & Ando, T. Video imaging of walking myosin V by high-speed atomic force microscopy. Nature 468, 72–76 (2010).
Acknowledgements
This publication was funded, in part, by the Engineering & Physical Sciences Research Council (EPSRC). L.P. & O.D.P. thank the Royal Academy of Engineering for funding the development of the HS-AFM and the NSQI Low Noise Lab for hosting the HS-AFM. We thank Martial Duchamp for his valuable assistance with the ‘PICO’ TEM at the Ernst–Ruska Research Centre. The authors are grateful to Milo Shaffer & Paul McMillan for helpful and supportive discussions.
Author information
Authors and Affiliations
Contributions
C.A.H. and P.L.C. conceived the project. P.L.C., V.T., M.K.B.S., K.M.C. and C.A.H. made the intercalated compounds and solutions. M.K.B.S., K.M.C., P.L.C. & C.A.H. carried out X-ray analysis. L.P., O.D.P., P.L.C., K.M.C. and C.A.H. performed and analysed the AFM measurements. L.P. wrote the automated HS-AFM platelet detection and step height measurement algorithms. V.T. performed S/TEM and contributed to the analysis of the results. C.A.H. carried out the electroplating and Raman experiment. C.A.H. directed the study and wrote the paper. All authors discussed and developed the science and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 13917 kb)
Supplementary movie
Supplementary movie 1 (MP4 14021 kb)
Rights and permissions
About this article
Cite this article
Cullen, P., Cox, K., Bin Subhan, M. et al. Ionic solutions of two-dimensional materials. Nature Chem 9, 244–249 (2017). https://doi.org/10.1038/nchem.2650
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2650
This article is cited by
-
Synthesis of atomically thin sheets by the intercalation-based exfoliation of layered materials
Nature Synthesis (2023)
-
High-yield production of mono- or few-layer transition metal dichalcogenide nanosheets by an electrochemical lithium ion intercalation-based exfoliation method
Nature Protocols (2022)
-
2D Semiconductor Nanomaterials and Heterostructures: Controlled Synthesis and Functional Applications
Nanoscale Research Letters (2021)
-
Observation of stress corrosion cracking using real-time in situ high-speed atomic force microscopy and correlative techniques
npj Materials Degradation (2021)
-
Exfoliating large monolayers in liquids
Nature Materials (2021)