Abstract
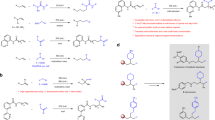
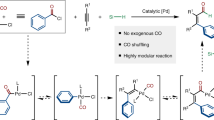
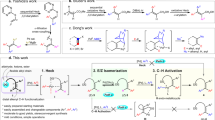
Regioselective activation of aromatic C–H bonds is a long-standing challenge for arene functionalization reactions such as the hydroarylation of alkynes. One possible solution is to employ a removable directing group that activates one of several aromatic C–H bonds. Here we report a new catalytic method for regioselective alkyne hydroarylation with benzoic acid derivatives during which the carboxylate functionality directs the alkyne to the ortho-C–H bond with elimination in situ to form a vinylarene product. The decarboxylation stage of this tandem sequence is envisioned to proceed with the assistance of an ortho-alkenyl moiety, which is formed by the initial alkyne coupling. This ruthenium-catalysed decarboxylative alkyne hydroarylation eliminates the common need for pre-existing ortho-substitution on benzoic acids for substrate activation, proceeds under redox-neutral and relatively mild conditions, and tolerates a broad range of synthetically useful aromatic functionality. Thus, it significantly increases the synthetic utility of benzoic acids as easily accessible aromatic building blocks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jia, C. et al. Efficient activation of aromatic C–H bonds for addition to C–C multiple bonds. Science 287, 1992–1995 (2000).
Nevado, C. & Echavarren, A. M. Transition metal-catalyzed hydroarylation of alkynes. Synthesis, 167–182 (2005).
Mitchell, D. & Yu, H. Synthetic applications of palladium-catalyzed hydroarylation and related systems. Curr. Opin. Drug Discov. Dev. 6, 876–883 (2003).
Kitamura, T. Transition-metal-catalyzed hydroarylation reactions of alkynes through direct functionalization of C–H bonds. A convenient tool for organic synthesis. Eur. J. Org. Chem. 1111–1125 (2009).
Bandini, M. Gold-catalyzed decorations of arenes and heteroarenes with C–C multiple bonds. Chem. Soc. Rev. 40, 1358–1367 (2011).
Cacchi, S. The palladium-catalyzed hydroarylation and hydrovinylation of carbon–carbon multiple bonds: new perspectives in organic synthesis. Pure Appl. Chem. 62, 713–722 (1990).
Cacchi, S., Fabrizi, G., Goggiamani, A. & Persiani, D. Palladium-catalyzed hydroarylation of alkynes with arenediazonium salts. Org. Lett. 10, 1597–1600 (2008).
Xu, X. et al. Palladium-catalyzed hydroarylation of alkynes with arylboronic acids. Tetrahedron 66, 2433–2438 (2010).
Song, C. E., Jun, D.-U., Choung, S.-Y., Roh, E. J. & Lee, S.-G. Dramatic enhancement of catalytic activity in an ionic liquid: highly practical Friedel–Crafts alkenylation of arenes with alkynes catalyzed by metal triflates. Angew. Chem. Int. Ed. 43, 6183–6185 (2004).
Li, R., Wang, S. R. & Lu, W. FeCl3-catalyzed alkenylation of simple arenes with aryl-substituted alkynes. Org. Lett. 9, 2219–2222 (2007).
Reetz, M. T. & Sommer, K. Gold-catalyzed hydroarylation of alkynes. Eur. J. Org. Chem. 3485–3496 (2003).
Shi, Z. & He, C. Efficient functionalization of aromatic C–H bonds catalyzed by gold(III) under mild and solvent-free conditions. J. Org. Chem. 69, 3669–3671 (2004).
Albrecht, M. Cyclometalation using d-block transition metals: fundamental aspects and recent trends. Chem. Rev. 110, 576–623 (2010).
Lewis, L. N. & Smith, J. F. Catalytic C–C bond formation via ortho-metalated complexes. J. Am. Chem. Soc. 108, 2728–2735 (1986).
Murai, S. et al. Efficient catalytic addition of aromatic carbon–hydrogen bonds to olefins. Nature 366, 529–531 (1993).
Kakiuchi, F., Yamamoto, Y., Chatani, N. & Murai, S. Catalytic addition of aromatic C–H bonds to acetylenes. Chem. Lett. 681–682 (1995).
Schipper, D. J., Hutchinson, M. & Fagnou, K. Rhodium(III)-catalyzed intermolecular hydroarylation of alkynes. J. Am. Chem. Soc. 132, 6910–6911 (2010).
Hashimoto, Y., Hirano, K., Satoh, T., Kakiuchi, F. & Miura, M. Ruthenium(II)-catalyzed regio- and stereoselective hydroarylation of alkynes via directed C–H functionalization. Org. Lett. 14, 2058–2061 (2012).
Hashimoto, Y., Hirano, K., Satoh, T., Kakiuchi, F. & Miura, M. Regioselective C–H bond cleavage/alkyne insertion under ruthenium catalysis. J. Org. Chem. 78, 638–646 (2013).
Min, M., Kim, D. & Hong, S. AgSbF6-controlled diastereodivergence in alkyne hydroarylation: facile access to Z- and E-alkenyl arenes. Chem. Commun. 50, 8028–8031 (2014).
Zhang, F. & Spring, D. R. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 43, 6906–6919 (2014).
Rousseau, G. & Breit, B. Removable directing groups in organic synthesis and catalysis. Angew. Chem. Int. Ed. 50, 2450–2494 (2011).
Liu, G., Shen, Y., Zhou, Z. & Lu, X. Rhodium(III)-catalyzed redox-neutral coupling of N-phenoxyacetamides and alkynes with tunable selectivity. Angew. Chem. Int. Ed. 52, 6033–6037 (2013).
Wang, C., Chen, H., Wang, Z., Chen, J. & Huang, Y. Rhodium(III)-catalyzed C–H activation of arenes using a versatile and removable triazene directing group. Angew. Chem. Int. Ed. 51, 7242–7245 (2012).
Tang, R.-Y., Li, G. & Yu, J.-Q. Conformation-induced remote meta-C–H activation of amines. Nature 507, 215–220 (2014).
Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev. 111, 1315–1345 (2011).
Shepard, A. F., Winslow, N. R. & Johnson, J. R. The simple halogen derivative of furan. J. Am. Chem. Soc. 52, 2083–2090 (1930).
Gooβen, L. J., Rodriguez, N. & Gooβen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 47, 3100–3120 (2008).
Rodriguez, N. & Gooβen, L. J. Decarboxylative coupling reactions: a modern strategy for C–C-bond formation. Chem. Soc. Rev. 40, 5030–5048 (2011).
Cornella, J. & Larrosa, I. Decarboxylative carbon–carbon bond-forming transformations of (hetero)aromatic carboxylic acids. Synthesis 44, 653–676 (2012).
Tang, J., Biafora, A. & Goossen, L. J. Catalytic decarboxylative cross-coupling of aryl chlorides and benzoates without activating ortho substituents. Angew. Chem. Int. Ed. 44, 13130–13133 (2015).
Myers, A. G., Tanaka, D. & Mannion, M. R. Development of a decarboxylative palladation reaction and its use in a Heck-type olefination of arene carboxylates. J. Am. Chem. Soc. 124, 11250–11251 (2002).
Gooβen, L. J., Deng, G. & Levy, L. M. Decarboxylation: synthesis of biaryls via catalytic decarboxylative coupling. Science 313, 662–664 (2006).
Gooβen, L. J., Thiel, W. R., Rodriguez, N., Linder, C. & Melzer, B. Copper-catalyzed protodecarboxylation of aromatic carboxylic acids. Adv. Synth. Catal. 349, 2241–2246 (2007).
Ueura, K., Satoh, T. & Miura, M. Rhodium- and iridium-catalyzed oxidative coupling of benzoic acids with alkynes via regioselective C–H bond cleavage. J. Org. Chem. 72, 5362–5367 (2007).
Maehara, A., Tsurugi, H., Satoh, T. & Miura, M. Regioselective C–H functionalization directed by a removable carboxyl group: palladium-catalyzed vinylation at the unusual position of indole and related heteroaromatic rings. Org. Lett. 10, 1159–1162 (2008).
Mochida, S., Hirano, K., Satoh, T. & Miura, M. Synthesis of stilbene and distyrylbenzene derivatives through rhodium-catalyzed ortho-olefination and decarboxylation of benzoic acids. Org. Lett. 12, 5776–5779 (2010).
Wang, C., Rakshit, S. & Glorius, F. Palladium-catalyzed intermolecular decarboxylative coupling of 2-phenylbenzoic acids with alkynes via C–H and C–C bond activation. J. Am. Chem. Soc. 132, 14006–14008 (2010).
Cornella, J., Righi, M. & Larrosa, I. Carboxylic acids as traceless directing groups for formal meta-selective direct arylation. Angew. Chem. Int. Ed. 50, 9429–9432 (2011).
Bhadra, S., Dzik, W. I. & Gooβen, L. J. Synthesis of aryl ethers from benzoates through carboxylate-directed C–H-activating alkoxylation with concomitant protodecarboxylation. Angew. Chem. Int. Ed. 52, 2959–2962 (2013).
Mamone, P., Danoun, G. & Gooβen, L. J. Rhodium-catalyzed ortho acylation of aromatic carboxylic acids. Angew. Chem. Int. Ed. 52, 6704–6708 (2013).
Luo, J., Preciado, S. & Larrosa, I. Overriding ortho−para selectivity via a traceless directing group relay strategy: the meta-selective arylation of phenols. J. Am. Chem. Soc. 136, 4109–4112 (2014).
Quan, Y. & Xie, Z. Iridium catalyzed regioselective cage boron alkenylation of o-carboranes via direct cage B−H activation. J. Am. Chem. Soc. 136, 15513–15516 (2014).
Qin, X., Sun, D., You, Q., Cheng, Y. & You, J. Rh(III)-catalyzed decarboxylative ortho-heteroarylation of aromatic carboxylic acids by using the carboxylic acid as a traceless directing group. Org. Lett. 17, 1762–1765 (2015).
Zhang, Y., Zhao, H., Zhang, M. & Su, W. Carboxylic acids as traceless directing groups for the rhodium(III)-catalyzed decarboxylative C–H arylation of thiophenes. Angew. Chem. Int. Ed. 54, 3817–3821 (2015).
Shi, X.-Y. et al. A convenient synthesis of N-aryl benzamides by rhodium-catalyzed ortho-amidation and decarboxylation of benzoic acids. Chem. Eur. J. 21, 1900–1903 (2015).
Shi, X.-Y. et al. Ru(II)-catalyzed ortho-amidation and decarboxylation of aromatic acids: a versatile route to meta-substituted N-aryl benzamides. Sci. China Chem. 58, 1286–1291 (2015).
Ackermann, L., Pospech, J., Graczyk, K. & Rauch, K. Versatile synthesis of isocoumarins and α-pyrones by ruthenium-catalyzed oxidative C–H/O–H bond cleavages. Org. Lett. 14, 930–933 (2012).
Arockiam, P. B., Bruneau, C. & Dixneuf, P. H. Ruthenium(II)-catalyzed C−H bond activation and functionalization. Chem. Rev. 112, 5879–5919 (2012).
Cacchi, S., Felici, M. & Pietroni, B. The palladium-catalyzed reaction of aryl iodides with mono- and disubstituted acetylenes: a new synthesis of trisubstituted alkenes. Tetrahedron Lett. 25, 3137–3140 (1984).
Acknowledgements
We thank the National Science Foundation (CHE-1301409 and RII-1330840 in association with the North Dakota Experimental Program to Stimulate Competitive Research (ND EPSCoR) to P.Z. and J.Z.) and ND EPSCoR (EPS-0447679, fellowship to J.Z.) for their financial support. The work of J.F.H. and R.S. was supported by the Director, Office of Science, US Department of Energy, under Contract no. DE-AC02-05CH11231. We also thank A. Ugrinov for assistance with the X-ray diffraction data collection and analysis.
Author information
Authors and Affiliations
Contributions
J.Z. performed the experiments and data analysis. R.S. participated in the high-throughput screening experiments for catalyst development. J.Z., J.F.H. and P.Z. designed the catalytic sequence and developed the reaction conditions. P.Z. and J.F.H. prepared this manuscript with feedback from J.Z. and R.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 8009 kb)
Supplementary information
Crystallographic data for compound 9. (CIF 616 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Shrestha, R., Hartwig, J. et al. A decarboxylative approach for regioselective hydroarylation of alkynes. Nature Chem 8, 1144–1151 (2016). https://doi.org/10.1038/nchem.2602
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2602
This article is cited by
-
Diastereo- and atroposelective synthesis of N-arylpyrroles enabled by light-induced phosphoric acid catalysis
Nature Communications (2023)
-
Trisubstituted alkenes featuring aryl groups: stereoselective synthetic strategies and applications
Science China Chemistry (2023)
-
Ni-catalyzed hydroarylation of alkynes with unactivated β-C(sp2)−H bonds
Nature Communications (2022)
-
Merging C–H and C–C bond cleavage in organic synthesis
Nature Reviews Chemistry (2017)
-
Good things come in threes
Nature Chemistry (2016)