Abstract
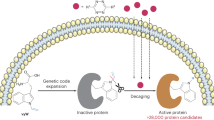
Using small molecules to control the function of proteins in live cells with complete specificity is highly desirable, but challenging. Here we report a small-molecule switch that can be used to control protein activity. The approach uses a phosphine-mediated Staudinger reduction to activate protein function. Genetic encoding of an ortho-azidobenzyloxycarbonyl amino acid using a pyrrolysyl transfer RNA synthetase/tRNACUA pair in mammalian cells enables the site-specific introduction of a small-molecule-removable protecting group into the protein of interest. Strategic placement of this group renders the protein inactive until deprotection through a bioorthogonal Staudinger reduction delivers the active wild-type protein. This developed methodology was applied to the conditional control of several cellular processes, including bioluminescence (luciferase), fluorescence (enhanced green fluorescent protein), protein translocation (nuclear localization sequence), DNA recombination (Cre) and gene editing (Cas9).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bishop, A. C. et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 (2000).
Buckley, D. L. & Crews, C. M. Small-molecule control of intracellular protein levels through modulation of the ubiquitin proteasome system. Angew. Chem. Int. Ed. 53, 2312–2330 (2014).
Winter, G. E. et al. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381 (2015).
Warner, J. B., Muthusamy, A. K. & Petersson, E. J. Specific modulation of protein activity by using a bioorthogonal reaction. Chembiochem 15, 2508–2514 (2014).
Buskirk, A. R. & Liu, D. R. Creating small-molecule-dependent switches to modulate biological functions. Chem. Biol. 12, 151–161 (2005).
Zorn, J. A. & Wells, J. A. Turning enzymes ON with small molecules. Nature Chem. Biol. 6, 179–188 (2010).
Putyrski, M. & Schultz, C. Protein translocation as a tool: the current rapamycin story. FEBS Lett. 586, 2097–2105 (2012).
Fegan, A., White, B., Carlson, J. C. & Wagner, C. R. Chemically controlled protein assembly: techniques and applications. Chem. Rev. 110, 3315–3336 (2010).
Rakhit, R., Navarro, R. & Wandless, T. J. Chemical biology strategies for posttranslational control of protein function. Chem. Biol. 21, 1238–1252 (2014).
Ho, S. N., Biggar, S. R., Spencer, D. M., Schreiber, S. L. & Crabtree, G. R. Dimeric ligands define a role for transcriptional activation domains in reinitiation. Nature 382, 822–826 (1996).
Gray, D. C., Mahrus, S. & Wells, J. A. Activation of specific apoptotic caspases with an engineered small-molecule-activated protease. Cell 142, 637–646 (2010).
Karginov, A. V., Ding, F., Kota, P., Dokholyan, N. V. & Hahn, K. M. Engineered allosteric activation of kinases in living cells. Nature Biotechnol. 28, 743–747 (2010).
Jullien, N., Sampieri, F., Enjalbert, A. & Herman, J. P. Regulation of Cre recombinase by ligand-induced complementation of inactive fragments. Nucleic Acids Res. 31, e131 (2003).
Bonger, K. M., Chen, L. C., Liu, C. W. & Wandless, T. J. Small-molecule displacement of a cryptic degron causes conditional protein degradation. Nature Chem. Biol. 7, 531–537 (2011).
Chu, P. H. et al. Engineered kinase activation reveals unique morphodynamic phenotypes and associated trafficking for Src family isoforms. Proc. Natl Acad. Sci. USA 111, 12420–12425 (2014).
Miyamoto, T. et al. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nature Chem. Biol. 8, 465–470 (2012).
Gautier, A. et al. How to control proteins with light in living systems. Nature Chem. Biol. 10, 533–541 (2014).
Weitzman, M. & Hahn, K. M. Optogenetic approaches to cell migration and beyond. Curr. Opin. Cell Biol. 30, 112–120 (2014).
Chen, X. et al. Synthetic dual-input mammalian genetic circuits enable tunable and stringent transcription control by chemical and light. Nucleic Acids Res. 44, 2677–2690 (2016).
Jeong, J. W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Li, J., Jia, S. & Chen, P. R. Diels–Alder reaction-triggered bioorthogonal protein decaging in living cells. Nature Chem. Biol. 10, 1003–1005 (2014).
Hemphill, J., Chou, C. J., Chin, J. W. & Deiters, A. Genetically encoded light-activated transcription for spatiotemporal control of gene expression and gene silencing in mammalian cells. J. Am. Chem. Soc. 135, 13433–13439 (2013).
Li, H., Hah, J. M. & Lawrence, D. S. Light-mediated liberation of enzymatic activity: ‘small molecule’ caged protein equivalents. J. Am. Chem. Soc. 130, 10474–10475 (2008).
Li, J. et al. Palladium-triggered deprotection chemistry for protein activation in living cells. Nature Chem. 6, 352–361 (2014).
Vila-Perello, M., Hori, Y., Ribo, M. & Muir, T. W. Activation of protein splicing by protease- or light-triggered O to N acyl migration. Angew. Chem. Int. Ed. 47, 7764–7767 (2008).
Sainlos, M., Iskenderian-Epps, W. S., Olivier, N. B., Choquet, D. & Imperiali, B. Caged mono- and divalent ligands for light-assisted disruption of PDZ domain-mediated interactions. J. Am. Chem. Soc. 135, 4580–4583 (2013).
Kohn, M. & Breinbauer, R. The Staudinger ligation—a gift to chemical biology. Angew. Chem. Int. Ed. 43, 3106–3116 (2004).
Van Berkel, S. S., Van Eldijk, M. B. & Van Hest, J. C. Staudinger ligation as a method for bioconjugation. Angew. Chem. Int. Ed. 50, 8806–8827 (2011).
Sletten, E. M. & Bertozzi, C. R. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc. Chem. Res. 44, 666–676 (2011).
Alouane, A., Labruere, R., Le Saux, T., Schmidt, F. & Jullien, L. Self-immolative spacers: kinetic aspects, structure–property relationships, and applications. Angew. Chem. Int. Ed. 54, 7492–7509 (2015).
Chen, P. R. et al. A facile system for encoding unnatural amino acids in mammalian cells. Angew. Chem. Int. Ed. 48, 4052–4055 (2009).
Takimoto, J. K., Dellas, N., Noel, J. P. & Wang, L. Stereochemical basis for engineered pyrrolysyl-tRNA synthetase and the efficient in vivo incorporation of structurally divergent non-native amino acids. ACS Chem. Biol. 6, 733–743 (2011).
Hancock, S. M., Uprety, R., Deiters, A. & Chin, J. W. Expanding the genetic code of yeast for incorporation of diverse unnatural amino acids via a pyrrolysyl-tRNA synthetase/tRNA pair. J. Am. Chem. Soc. 132, 14819–14824 (2010).
Plass, T., Milles, S., Koehler, C., Schultz, C. & Lemke, E. A. Genetically encoded copper-free click chemistry. Angew. Chem. Int. Ed. 50, 3878–3881 (2011).
Wang, Y. S., Fang, X. Q., Wallace, A. L., Wu, B. & Liu, W. S. R. A rationally designed pyrrolysyl-tRNA synthetase mutant with a broad substrate spectrum. J. Am. Chem. Soc. 134, 2950–2953 (2012).
Yanagisawa, T. et al. Multistep engineering of pyrrolysyl-tRNA synthetase to genetically encode Nε-(o-azidobenzyloxycarbonyl) lysine for site-specific protein modification. Chem. Biol. 15, 1187–1197 (2008).
Luo, J. et al. Genetically encoded optochemical probes for simultaneous fluorescence reporting and light activation of protein function with two-photon excitation. J. Am. Chem. Soc. 136, 15551–15558 (2014).
Iizuka, R., Yamagishi-Shirasaki, M. & Funatsu, T. Kinetic study of de novo chromophore maturation of fluorescent proteins. Anal. Biochem. 414, 173–178 (2011).
Kawai, K. et al. A reductant-resistant and metal-free fluorescent probe for nitroxyl applicable to living cells. J. Am. Chem. Soc. 135, 12690–12696 (2013).
Saneyoshi, H. et al. Triphenylphosphinecarboxamide: an effective reagent for the reduction of azides and its application to nucleic acid detection. Org. Lett. 16, 30–33 (2014).
Cline, D. J. et al. New water-soluble phosphines as reductants of peptide and protein disulfide bonds: reactivity and membrane permeability. Biochemistry 43, 15195–15203 (2004).
Baker, A. S. & Deiters, A. Optical control of protein function through unnatural amino acid mutagenesis and other optogenetic approaches. ACS Chem. Biol. 9, 1398–1407 (2014).
Brieke, C., Rohrbach, F., Gottschalk, A., Mayer, G. & Heckel, A. Light-controlled tools. Angew. Chem. Int. Ed. 51, 8446–8476 (2012).
Engelke, H., Chou, C., Uprety, R., Jess, P. & Deiters, A. Control of protein function through optochemical translocation. ACS Synth. Biol. 3, 731–736 (2014).
Ho, P. S. & Eichman, B. F. The crystal structures of DNA Holliday junctions. Curr. Opin. Struct. Biol. 11, 302–308 (2001).
Nagy, A. Cre recombinase: the universal reagent for genome tailoring. Genesis 26, 99–109 (2000).
Gibb, B. et al. Requirements for catalysis in the Cre recombinase active site. Nucleic Acids Res. 38, 5817–5832 (2010).
Yang, Y. S. & Hughes, T. E. Cre stoplight: a red/green fluorescent reporter of Cre recombinase expression in living cells. Biotechniques 31, 1036–1041 (2001).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Davis, K. M., Pattanayak, V., Thompson, D. B., Zuris, J. A. & Liu, D. R. Small molecule-triggered Cas9 protein with improved genome-editing specificity. Nature Chem. Biol. 11, 316–318 (2015).
Zetsche, B., Volz, S. E. & Zhang, F. A split-Cas9 architecture for inducible genome editing and transcription modulation. Nature Biotechnol. 33, 139–142 (2015).
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156, 935–949 (2014).
Hemphill, J., Borchardt, E. K., Brown, K., Asokan, A. & Deiters, A. Optical control of CRISPR/Cas9 gene editing. J. Am. Chem. Soc. 137, 5642–5645 (2015).
Acknowledgements
This research was supported in part by the National Institutes of Health (1R01GM112728), the National Science Foundation (MCB-1330746) and the Charles E. Kaufman Foundation of The Pittsburgh Foundation. K.M. is grateful for a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad. We thank the Chin lab (Medical Research Council) for plasmids encoding the PylRS and PylT genes, the Asokan lab (University of North Carolina) for the pgRNA and pIRG plasmids and the Hughes lab (Montana State University) for the Cre Stoplight plasmid.
Author information
Authors and Affiliations
Contributions
A.D. and J.L. conceived and designed the experiments. J.L, Q.L. and K.M. performed the experiments and analysed the data. A.D. and J.L. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2316 kb)
Supplementary information
Supplementary Movie 1 (MP4 2417 kb)
Supplementary information
Supplementary Movie 2 (MP4 999 kb)
Rights and permissions
About this article
Cite this article
Luo, J., Liu, Q., Morihiro, K. et al. Small-molecule control of protein function through Staudinger reduction. Nature Chem 8, 1027–1034 (2016). https://doi.org/10.1038/nchem.2573
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2573
This article is cited by
-
Metal-responsive regulation of enzyme catalysis using genetically encoded chemical switches
Nature Communications (2022)
-
Bioorthogonal chemistry
Nature Reviews Methods Primers (2021)
-
Developing bioorthogonal probes to span a spectrum of reactivities
Nature Reviews Chemistry (2020)
-
Conditional control of RNA-guided nucleic acid cleavage and gene editing
Nature Communications (2020)
-
Site-specific ubiquitylation and SUMOylation using genetic-code expansion and sortase
Nature Chemical Biology (2019)