Abstract
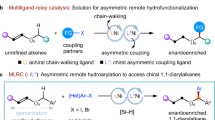
Amines with remote stereocentres (stereocentres that are three or more bonds away from the C–N bond) are important structural elements in many pharmaceutical agents and natural products. However, previously reported methods to prepare these compounds in an enantioselective manner are indirect and require multistep synthesis. Here, we report a copper-hydride-catalysed, enantioselective synthesis of γ- or δ-chiral amines from readily available allylic alcohols, esters and ethers using a reductive relay hydroamination strategy (a net reductive process in which an amino group is installed at a site remote from the original carbon–carbon double bond). The protocol was suitable for substrates containing a wide range of functional groups and provided remote chiral amine products with high levels of regio- and enantioselectivity. Sequential amination of substrates containing several carbon–carbon double bonds could be achieved, demonstrating the high chemoselectivity of this process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley-VCH, 2010).
Nguyen, L. A., He, H. & Pham-Huy, C. Chiral drugs: an overview. Int. J. Biomed. Sci. 2, 85–100 (2006).
Werner, E. W., Mei, T.-S., Burckle, A. J. & Sigman, M. S. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012).
Mei, T.-S., Patel, H. H. & Sigman, M. S. Enantioselective construction of remote quaternary stereocenters. Nature 508, 340–344 (2014).
Pirnot, M. T., Rankic, D. A., Martin, D. B. C. & MacMillan, D. W. C. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science 339, 1593–1596 (2013).
Deutsch, C., Krause, N. & Lipshutz, B. H. CuH-catalyzed reactions. Chem. Rev. 108, 2916–2927 (2008).
Lipshutz, B. H. Rediscovering organocopper chemistry through copper hydride. It's all about the ligand. Synlett 509–524 (2009).
Alexakis, A., Krause, N. & Woodward, S. Copper-Catalyzed Asymmetric Synthesis (Wiley, 2014).
Ketcham, J. M., Shin, I., Montgomery, T. P. & Krische, M. J. Catalytic enantioselective C−H functionalization of alcohols by redox-triggered carbonyl addition: borrowing hydrogen, returning carbon. Angew. Chem. Int. Ed. 53, 9142–9150 (2014).
Gui, J. et al. Practical olefin hydroamination with nitroarenes. Science 348, 886–891 (2015).
Sahli, Z., Sundararaju, B., Achard, M. & Bruneau, C. Ruthenium-catalyzed reductive amination of allylic alcohols. Org. Lett. 13, 3964–3967 (2011).
Li, H., Achard, M., Bruneau, C., Sortais, J.-B. & Darcel, C. Iron-catalysed tandem isomerisation/hydrosilylation reaction of allylic alcohols with amines. RSC Adv. 4, 25892–25897 (2014).
Müller, T. E., Hultzch, K. C., Yus, M., Foubelo, F. & Tada, M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 108, 3795–3892 (2008).
Hesp, K. D. Copper-catalyzed regio- and enantioselective hydroamination of alkenes with hydroxylamines. Angew. Chem. Int. Ed. 53, 2034–2036 (2014).
Huang, L., Arndt, M., Gooßen, K., Heydt, H. & Gooßen, L. J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 115, 2596–2697 (2015).
Zhu, S., Niljianskul, N. & Buchwald, S. L. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J. Am. Chem. Soc. 135, 15746–15749 (2013).
Niljianskul, N., Zhu, S. & Buchwald, S. L. Enantioselective synthesis of α-aminosilanes by copper-catalyzed hydroamination of vinylsilanes. Angew. Chem. Int. Ed. 54, 1638–1641 (2015).
Shi, S.-L. & Buchwald, S. L. Copper-catalysed selective hydroamination reactions of alkynes. Nature Chem. 7, 38–44 (2015).
Niu, D. & Buchwald, S. L. The design of modified amine transfer reagents allows the synthesis of α-chiral secondary amines via CuH-catalyzed hydroamination. J. Am. Chem. Soc. 137, 9716–9721 (2015).
Miki, Y., Hirano, K., Satoh, T. & Miura, M. Copper-catalyzed intermolecular regioselective hydroamination of styrenes with polymethylhydrosiloxane and hydroxylamines. Angew. Chem. Int. Ed. 52, 10830–10834 (2013).
Miki, Y., Hirano, K., Satoh, T. & Miura, M. Copper-catalyzed enantioselective formal hydroamination of oxa- and azabicyclic alkenes with hydrosilanes and hydroxylamines. Org. Lett. 16, 1498–1501 (2014).
Zhu, S. & Buchwald, S. L. Enantioselective CuH-catalyzed anti-Markovnikov hydroamination of 1,1-disubstituted alkenes. J. Am. Chem. Soc. 136, 15913–15916 (2014).
Yorimitsu, H. & Oshima, K. Recent progress in asymmetric allylic substitutions catalyzed by chiral copper complexes. Angew. Chem. Int. Ed. 44, 4435–4439 (2005).
Alexakis, A., Bäckvall, J. E., Krause, N., Pàmies, O. & Diéguez, M. Enantioselective copper-catalyzed conjugate addition and allylic substitution reactions. Chem. Rev. 108, 2796–2823 (2008).
Ito, H., Ito, S., Sasaki, Y., Matsuura, K. & Sawamura, M. Copper-catalyzed enantioselective substitution of allylic carbonates with diboron: an efficient route to optically active α-chiral allylboronates. J. Am. Chem. Soc. 129, 14856–14857 (2007).
Park, J. K., Lackey, H. H., Ondrusek, B. A. & McQuade, D. T. Stereoconvergent synthesis of chiral allylboronates from an E/Z mixture of allylic aryl ethers using a 6-NHC-Cu(I) catalyst. J. Am. Chem. Soc. 133, 2410–2413 (2011).
Guzman-Martinez, A. & Hoveyda, A. H. Enantioselective synthesis of allylboronates bearing a tertiary or quaternary β-substituted stereogenic carbon by NHC-Cu-catalyzed substitution reactions. J. Am. Chem. Soc. 132, 10634–10637 (2010).
Ito, H., Kunii, S. & Sawamura, M. Direct enantio-convergent transformation of racemic substrates without racemization or symmetrization. Nature Chem. 2, 972–976 (2010).
Delvos, L. B., Vyas, D. J. & Oestreich, M. Asymmetric synthesis of α-chiral allylic silanes by enantioconvergent γ-selective copper(I)-catalyzed allylic silylation. Angew. Chem. Int. Ed. 52, 4650–4653 (2013).
Brestensky, D. M. & Stryker, J. M. Regioselective conjugate reduction and reductive silylation of α,β-unsaturated. Tetrahedron Lett. 30, 5677–5680 (1989).
Lipshutz, B. H., Chrisman, W. & Noson, K. Hydrosilylation of aldehydes and ketones catalyzed by [Ph3P(CuH)]6 . J. Org. Chem. 624, 367–371 (2001).
Lipshutz, B. H., Noson, K., Chrisman, W. & Lower, A. Asymmetric hydrosilylation of aryl ketones catalyzed by copper hydride complexed by nonracemic biphenyl bis-phosphine ligands. J. Am. Chem. Soc. 125, 8779–8782 (2003).
Acknowledgements
This paper is dedicated to P. Knochel on the occasion of his 60th birthday. Research reported in this publication was supported by the National Institutes of Health under award no. GM58160. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank P. Müller (MIT) for X-ray analysis of 3g and Y.-M. Wang, M.T. Pirnot and C. Nguyen for their advice on the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S.Z. and S.L.B designed the project. S.Z., N.N. and S.L.B. co-wrote the manuscript, analysed the data, discussed the results and commented on the manuscript. S.Z. and N.N. performed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5615 kb)
Supplementary information
Crystallographic data for compound 3g. (CIF 1225 kb)
Rights and permissions
About this article
Cite this article
Zhu, S., Niljianskul, N. & Buchwald, S. A direct approach to amines with remote stereocentres by enantioselective CuH-catalysed reductive relay hydroamination. Nature Chem 8, 144–150 (2016). https://doi.org/10.1038/nchem.2418
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2418
This article is cited by
-
Structural transformation and catalytic hydrogenation activity of amidinate-protected copper hydride clusters
Nature Communications (2022)
-
Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)-H functionalization
Nature Communications (2021)
-
Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines
Nature Communications (2021)
-
Rhodium-catalyzed intermolecular enantioselective Alder–ene type reaction of cyclopentenes with silylacetylenes
Nature Communications (2021)
-
Copper-hydride nanoclusters with enhanced stability by N-heterocyclic carbenes
Nano Research (2021)