Abstract
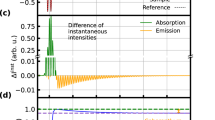
The role of vibrational coherence—concerted vibrational motion on the excited-state potential energy surface—in the isomerization of retinal in the protein rhodopsin remains elusive, despite considerable experimental and theoretical efforts. We revisited this problem with resonant ultrafast heterodyne-detected transient-grating spectroscopy. The enhanced sensitivity that this technique provides allows us to probe directly the primary photochemical reaction of vision with sufficient temporal and spectral resolution to resolve all the relevant nuclear dynamics of the retinal chromophore during isomerization. We observed coherent photoproduct formation on a sub-50 fs timescale, and recovered a host of vibrational modes of the retinal chromophore that modulate the transient-grating signal during the isomerization reaction. Through Fourier filtering and subsequent time-domain analysis of the transient vibrational dynamics, the excited-state nuclear motions that drive the isomerization reaction were identified, and comprise stretching, torsional and out-of-plane wagging motions about the local C11=C12 isomerization coordinate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ernst, O. P. et al. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem. Rev. 114, 126–163 (2014).
Frutos, L. M., Andruniów, T., Santoro, F., Ferré, N. & Olivucci, M. Tracking the excited-state time evolution of the visual pigment with multiconfigurational quantum chemistry. Proc. Natl Acad. Sci. USA 104, 7764–7769 (2007).
Dartnall, H. J. A. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 8, 339–358 (1968).
Schoenlein, R. W., Peteanu, L. A., Mathies, R. A. & Shank, C. V. The first step in vision: femtosecond isomerization of rhodopsin. Science 254, 412–415 (1991).
Peteanu, L. A., Schoenlein, R. W., Wang, Q., Mathies, R. A. & Shank, C. V. The first step in vision occurs in femtoseconds: complete blue and red spectral studies. Proc. Natl Acad. Sci. USA 90, 11762–11766 (1993).
Yoshizawa, T. & Wald, G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature 197, 1279–1286 (1963).
Busch, G. E., Applebury, M. L., Lamola, A. A. & Rentzepis, P. M. Formation and decay of prelumirhodopsin at room temperature. Proc. Natl Acad. Sci. USA 69, 2802–2806 (1972).
Wang, Q., Schoenlein, R. W., Peteanu, L. A., Mathies, R. A. & Shank, C. V. Vibrationally coherent photochemistry in the femtosecond primary event of vision. Science 266, 422–424 (1994).
Eyring, G., Curry, B., Broek, A., Lugtenburg, J. & Mathies, R. A. Assignment and interpretation of hydrogen out-of-plane vibrations in the resonance Raman spectra of rhodopsin and bathorhodopsin. Biochemistry 21, 384–393 (1982).
Palings, I. et al. Assignment of fingerprint vibrations in the resonance Raman spectra of rhodopsin, isorhodopsin, and bathorhodopsin: implications for chromophore structure and environment. Biochemistry 26, 2544–2556 (1987).
Lin, S. W. et al. vibrational assignment of torsional normal modes of rhodopsin: probing excited-state isomerization dynamics along the reactive C11=C12 torsion coordinate. J. Phys. Chem. B 40, 2787–2806 (1998).
Kim, J. E., Tauber, M. J. & Mathies, R. A. Wavelength dependent cis–trans isomerization in vision. Biochemistry 40, 13774–13778 (2001).
Kim, J. E., Tauber, M. J. & Mathies, R. A. Analysis of the mode-specific excited-state energy distribution and wavelength-dependent photoreaction quantum yield in rhodopsin. Biophys. J. 84, 2492–2501 (2003).
Kukura, P., McCamant, D. W., Yoon, S., Wandschneider, D. B. & Mathies, R. A. Structural observation of the primary isomerization in vision with femtosecond-stimulated Raman. Science 310, 1006–1009 (2005).
Weingart, O. et al. Product formation in rhodopsin by fast hydrogen motions. Phys. Chem. Chem. Phys. 13, 3645–3648 (2011).
Schapiro, I. et al. The ultrafast photoisomerizations of rhodopsin and bathorhodopsin are modulated by bond length alternation and HOOP driven electronic effects. J. Am. Chem. Soc. 133, 3354–3364 (2011).
Polli, D. et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 467, 440–443 (2010).
Kahan, A., Nahmias, O., Friedman, N., Sheves, M. & Ruhman, S. Following photoinduced dynamics in bacteriorhodopsin with 7-fs impulsive vibrational spectroscopy. J. Am. Chem. Soc. 129, 537–546 (2007).
Yabushita, A. & Kobayashi, T. Primary conformation change in bacteriorhodopsin on photoexcitation. Biophys. J. 96, 1447–1461 (2009).
Kraack, J. P., Buckup, T., Hampp, N. & Motzkus, M. Ground- and excited-state vibrational coherence dynamics in bacteriorhodopsin probed with degenerate four-wave-mixing experiments. ChemPhysChem 12, 1851–1859 (2011).
Liebel, M. et al. Direct observation of the coherent nuclear response after the absorption of a photon. Phys. Rev. Lett. 112, 238301 (2014).
Johnson, P. J. M. et al. The photocycle and ultrafast vibrational dynamics of bacteriorhodopsin in lipid nanodiscs. Phys. Chem. Chem. Phys. 16, 21310–21320 (2014).
Zhu, J. et al. Comparing photochemistry of n- and tert-butylamine all-trans retinal protonated Schiff-base: effects of C=N configurational inhomogeneity. Chem. Phys. Lett. 479, 229–233 (2009).
Kraack, J. P., Buckup, T. & Motzkus, M. Vibrational analysis of excited and ground electronic states of all-trans retinal protonated Schiff-bases. Phys. Chem. Chem. Phys. 13, 21402–21410 (2011).
Kraack, J. P., Buckup, T. & Motzkus, M. Evidence for the two-state-two-mode model in retinal protonated Schiff-bases from pump degenerate four-wave-mixing experiments. Phys. Chem. Chem. Phys. 14, 13979–13988 (2012).
Yabushita, A., Kobayashi, T. & Tsuda, M. Time-resolved spectroscopy of ultrafast photoisomerization of octopus rhodopsin under photoexcitation. J. Phys. Chem. B 116, 1920–1926 (2012).
Dexheimer, S. L. et al. Femtosecond impulsive excitation of nonstationary vibrational states in bacteriorhodopsin. Chem. Phys. Lett. 188, 61–66 (1992).
Pollard, W. T. et al. Theory of dynamic absorption spectroscopy of nonstationary states. 4. Application to 12-fs resonant impulsive Raman spectroscopy of bacteriorhodopsin. J. Phys. Chem. 96, 6147–6158 (1992).
Schnedermann, C., Liebel, M. & Kukura, P. Mode-specificity of vibrationally coherent internal conversion in rhodopsin during the primary visual event. J. Am. Chem. Soc. 137, 2886–2891 (2015).
Kovalenko, S. A., Dobryakov, A. L., Ruthmann, J. & Ernsting, N. P. Femtosecond spectroscopy of condensed phases with chirped supercontinuum probing. Phys. Rev. A 59, 2369–2384 (1999).
Goodno, G. D., Dadusc, G. & Miller, R. J. D. Ultrafast heterodyne-detected transient-grating spectroscopy using diffractive optics. J. Opt. Soc. Am. B 15, 1791–1794 (1998).
Maznev, A. A., Nelson, K. A. & Rogers, J. A. Optical heterodyne detection of laser-induced gratings. Opt. Lett. 23, 1319–1321 (1998).
Goodno, G. D. & Miller, R. J. D. Femtosecond heterodyne-detected four-wave-mixing studies of deterministic protein motions. 1. Theory and experimental technique of diffractive optics-based spectroscopy. J. Phys. Chem. A 103, 10619–10629 (1999).
Loppnow, G. R. & Mathies, R. A. Excited-state structure and isomerization dynamics of the retinal chromophore in rhodopsin from resonance Raman intensities. Biophys. J. 54, 35–43 (1988).
Kochendoerfer, G. G. & Mathies, R. A. Spontaneous emission study of the femtosecond isomerization dynamics of rhodopsin. J. Phys. Chem. 100, 14526–14532 (1996).
Kim, J. E. & Mathies, R. A. Anti-Stokes Raman study of vibrational cooling dynamics in the primary photochemistry of rhodopsin. J. Phys. Chem. A 106, 8508–8515 (2002).
Weingart, O. & Garavelli, M. Modelling vibrational coherence in the primary rhodopsin photoproduct. J. Chem. Phys. 137, 22A523 (2012).
Palczewski, K. et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 (2000).
Okada, T. et al. The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J. Mol. Biol. 342, 571–583 (2004).
Birge, R. R. & Pierce, B. M. A theoretical analysis of the two-photon properties of linear polyenes and the visual chromophores. J. Chem. Phys. 70, 165–178 (1979).
Becker, R. S. The visual process: photophysics and photoisomerization of model visual pigments and the primary reaction. Photochem. Photobiol. 48, 369–399 (1988).
Curry, B. et al. Vibrational analysis of the retinal isomers. Adv. Infrared Raman Spectrosc. 12, 115–178 (1985).
Garavelli, M., Celani, P., Berardi, F., Robb, M. A. & Olivucci, M. The protonated Schiff base: an ab initio minimal model for retinal photoisomerization. J. Am. Chem. Soc. 119, 6891–6901 (1997).
Hiyashi, S., Tajkhorshid, E. & Schulten, K. Photochemical reaction dynamics of the primary event of vision studied by means of a hybrid molecular simulation. Biophys. J. 96, 403–416 (2009).
Schaffer, H. E., Chance, R. R., Silbey, R. J., Knoll, K. & Schrock, R. R. Conjugation length dependence of Raman scattering in a series of linear polyenes: implications for polyacetylene. J. Chem. Phys. 94, 4161–4170 (1991).
Garavelli, M. et al. Photoisomerization path for a realistic retinal chromophore model: the nonatetraeniminium cation. J. Am. Chem. Soc. 120, 1285–1288 (1998).
Teller, E. The crossing of potential surfaces. J. Phys. Chem. 41, 109–116 (1937).
Ishii, K., Takeuchi, S. & Tahara, T. A 40-fs time-resolved absorption study on cis-stilbene in solution observation of wavepacket motion on the reactive excited state. Chem. Phys. Lett. 398, 400–406 (2004).
Ishii, K., Takeuchi, S. & Tahara, T. Pronounced non-Condon effects as the origin of the quantum beat observed in the time-resolved absorption signal from excited-state cis-stilbene. J. Phys. Chem. A 112, 2219–2227 (2008).
Takeuchi, S. et al. Spectroscopic tracking of structural evolution in ultrafast stilbene photoisomerization. Science 322, 1073–1077 (2008).
Prokhorenko, V. I. et al. Coherent control of retinal isomerization in bacteriorhodopsin. Science 313, 1257–1261 (2006).
Warshel, A. Bicycle-pedal model for the first step in the vision process. Nature 260, 679–683 (1976).
Liu, R. S. H. & Asato, A. E. The primary process of vision and the structure of bathorhodopsin: a mechanism for photoisomerization of polyenes. Proc. Natl Acad. Sci. USA 82, 259–263 (1937).
Papermaster, D. S. & Dreyer, W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry 13, 2638–2444 (1974).
Morizumi, T., Imai, H. & Shichida, Y. Direct observation of the complex formation of GDP-bound transducin with the rhodopsin intermediate having a visible absorption maximum in rod outer segment membranes. Biochemistry 44, 9936–9943 (2005).
Wald, G. & Brown, P. K. The molar extinction of rhodopsin. J. Gen. Physiol. 37, 189–200 (1953).
Johnson, P. J. M., Prokhorenko, V. I. & Miller, R. J. D. Enhanced bandwidth noncollinear optical parametric amplification with a narrowband anamorphic pump. Opt. Lett. 36, 2170–2172 (2011).
Prokhorenko, V. I., Halpin, A. & Miller, R. J. D. Coherently-controlled two-dimensional photon echo electronic spectroscopy. Opt. Express 17, 9764–9779 (1998).
Harris, F. J. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc. IEEE 66, 51–83 (1978).
Acknowledgements
P.J.M.J. thanks J.M. Morrow and B.S.W. Chang for many helpful discussions and shared laboratory equipment during the early stages of this work. L. Chen is acknowledged for excellent technical assistance. This research was supported by the Natural Sciences and Engineering Research Council of Canada (R.J.D.M.), the Max Planck Society (R.J.D.M.), the Canadian Institute for Advanced Research (R.J.D.M. and O.P.E.) and the Canada Excellence Research Chair program (O.P.E.). O.P.E. is the Anne & Max Tanenbaum Chair in Neuroscience at the University of Toronto.
Author information
Authors and Affiliations
Contributions
P.J.M.J., A.H. and V.I.P. constructed the instruments. T.M. prepared and characterized the sample. P.J.M.J. and A.H. performed the measurements. P.J.M.J and V.I.P. analysed the data. P.J.M.J. wrote the manuscript with editing from all authors. O.P.E. and R.J.D.M. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 940 kb)
Rights and permissions
About this article
Cite this article
Johnson, P., Halpin, A., Morizumi, T. et al. Local vibrational coherences drive the primary photochemistry of vision. Nature Chem 7, 980–986 (2015). https://doi.org/10.1038/nchem.2398
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2398
This article is cited by
-
Spin–vibronic coherence drives singlet–triplet conversion
Nature (2023)
-
Theory predicts UV/vis-to-IR photonic down conversion mediated by excited state vibrational polaritons
Nature Communications (2023)
-
Absolute excited state molecular geometries revealed by resonance Raman signals
Nature Communications (2022)
-
Direct structural observation of ultrafast photoisomerization dynamics in sinapate esters
Communications Chemistry (2022)
-
Quantum–classical simulations of rhodopsin reveal excited-state population splitting and its effects on quantum efficiency
Nature Chemistry (2022)