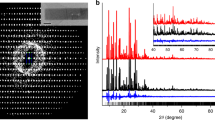
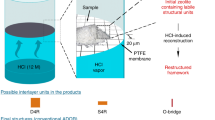
Abstract
Zeolites are porous aluminosilicate materials that have found applications in many different technologies. However, although simulations suggest that there are millions of possible zeolite topologies, only a little over 200 zeolite frameworks of all compositions are currently known, of which about 50 are pure silica materials. This is known as the zeolite conundrum—why have so few of all the possible structures been made? Several criteria have been formulated to explain why most zeolites are unfeasible synthesis targets. Here we demonstrate the synthesis of two such ‘unfeasible’ zeolites, IPC-9 and IPC-10, through the assembly–disassembly–organization–reassembly mechanism. These new high-silica zeolites have rare characteristics, such as windows that comprise odd-membered rings. Their synthesis opens up the possibility of preparing other zeolites that have not been accessible by traditional solvothermal synthetic methods. We envisage that these findings may lead to a step change in the number and types of zeolites available for future applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Akporiaye, D. E. & Price, G. D. Systematic enumeration of zeolite frameworks. Zeolites 9, 23–32 (1989).
Pophale, R., Cheeseman, P. A. & Deem, M. W. A database of new zeolite-like materials. Phys. Chem. Chem. Phys. 13, 12407–12412 (2011).
Blatov, V. A., Ilyushin, G. D. & Proserpio, D. M. The zeolite conundrum: why are there so many hypothetical zeolites and so few observed? A possible answer from the zeolite-type frameworks perceived as packings of tiles. Chem. Mater. 25, 412–424 (2013).
Cundy, C. S. & Cox, P. A. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem. Rev. 103, 663–701 (2003).
Cundy, C. S. & Cox, P. A. The hydrothermal synthesis of zeolites: precursors, intermediates and reaction mechanism. Micropor. Mesopor. Mater. 82, 1–78 (2005).
Foster, M. D. et al. Chemically feasible hypothetical crystalline networks. Nature Mater. 3, 234–238 (2004).
Sartbaeva, A., Wells, S. A., Treacy, M. M. J. & Thorpe, M. F. The flexibility window in zeolites. Nature Mater. 5, 962–965 (2006).
Li, Y., Yu, J. & Xu, R. R. Criteria for zeolite frameworks realizable for target synthesis. Angew. Chem. Int. Ed. 52, 1673–1677 (2013).
Akporiaye, D. E. & Price, G. D. Relative stability of zeolite frameworks from calculated energetics of known and theoretical structures. Zeolites 9, 321–328 (1989).
Henson, N. J., Cheetham, A. K. & Gale, J. D. Computational studies of aluminum phosphate polymorphs. Chem. Mater. 8, 664–670 (1996).
Henson, N. J., Cheetham, A. K. & Gale, J. D. Theoretical calculations on silica frameworks and their correlation with experiment. Chem. Mater. 6, 1647–1650 (1994).
Earl, D. J. & Deem, M. W. Toward a database of hypothetical zeolite structures. Ind. Eng. Chem. Res. 45, 5449–5454 (2006).
Li, X. & Deem, M. W. Why zeolites have so few seven-membered rings. J. Phys. Chem. C 118, 15835–15839 (2014).
Morris, R. E. & Cejka, J. Exploiting chemically selective weakness in solids as a route to new porous materials. Nature Chem. 7, 381–388 (2015).
Roth, W. J. et al. A family of zeolites with controlled pore size prepared using a top-down method. Nature Chem. 5, 628–633 (2013).
Wheatley, P. et al. Zeolites with continuously tuneable porosity. Angew. Chem. Int. Ed. 53, 13210–13214 (2014).
Chlubna-Eliasova, P. et al. The assembly–disassembly–organization–reassembly mechanism for 3D-2D-3D transformation of germanosilicate IWW zeolite. Angew. Chem. Int. Ed. 53, 7048–7052 (2014).
Roth, W. J., Nachtigall, P., Morris, R. E. & Cejka, J. Two-dimensional zeolites: current status and perspectives. Chem. Rev. 114, 4807–4837 (2014).
Paillaud, J. L., Harbuzaru, B., Patarin, J. & Bats, N. Extra-large-pore zeolites with two-dimensional channels formed by 14 and 12 rings. Science 304, 990–992 (2004).
Corma, A., Diaz-Cabanas, M. J., Rey, F., Nicolooulas, S. & Boulahya, K. ITQ-15: the first ultralarge pore zeolite with a bi-directional pore system formed by intersecting 14- and 12-ring channels, and its catalytic implications. Chem. Commun. 1356–1357 (2004).
Roth, W. J. et al. Postsynthesis transformation of three-dimensional framework into a lamellar zeolite with modifiable architecture. J. Am. Chem. Soc. 133, 6130–6133 (2011).
Grajciar, L., Bludsky, O., Roth, W. J. & Nachtigall, P. Theoretical investigation of layered zeolite frameworks: interaction between IPC-1P layers derived from zeolite UTL. Catal. Today 204, 15–21 (2013).
Trachta, M., Bludsky, O., Cejka, J., Morris, R. E. & Nachtigall, P. From double-four-ring germanosilicates to new zeolites: in silico investigation. ChemPhysChem 15, 2972–2976 (2014).
Trachta, M., Nachtigall, P. & Bludsky, O. The ADOR synthesis of new zeolites: in silico investigation. Catal. Today 243, 32–38 (2015).
Moliner, M., Martinez, C. & Corma, A. Multipore zeolites: synthesis and catalytic applications. Angew. Chem. Int. Ed. 54, 3560–3579 (2015).
Sanders, M., Leslie, M. & Catlow, C. Interatomic potentials for SiO2 . J. Chem. Soc. Chem. Commun. 1271–1273 (1984).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Acknowledgements
R.E.M. thanks the Royal Society and the Engineering and Physical Sciences Research Council (Grants EP/L014475/1, EP/K025112/1 and EP/K005499/1) for funding work in this area. J.Č. and P.N. acknowledge the Czech Science Foundation for the project of the Centre of Excellence (P106/12/G015) and the European Union Seventh Framework Programme (FP7/ 2007–2013) under Grant Agreement No. 604307. The research leading to these results has received funding from the European Union Seventh Framework Programme under Grant Agreement No. 312483—ESTEEM2 (Integrated Infrastructure Initiative–I3). W.J.R. thanks his current institution, Jagiellonian University in Krakow, Faculty of Chemistry. We thank W. Zhou and F. Yu for their expertise in TEM and D. Dawson for help with NMR spectroscopy.
Author information
Authors and Affiliations
Contributions
M.M., P.E. and W.J.R. completed the synthesis aspects of the work and P.S.W. coordinated the characterization of the prepared materials. M.P. completed the computational modelling under the supervision of P.N. M.N. and A.M. completed the aberration-corrected electron microscopy studies. J.Č. and R.E.M. coordinated the project as a whole and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2752 kb)
Supplementary information
Supplementary Movie 1 (MOV 6549 kb)
Supplementary information
Supplementary Movie 2 (MOV 9144 kb)
Supplementary information
Supplementary Movie 3 (MOV 12673 kb)
Supplementary information
Crystallographic data for compound IPC9 (CIF 22 kb)
Supplementary information
Crystallographic data for compound IPC10 (CIF 12 kb)
Rights and permissions
About this article
Cite this article
Mazur, M., Wheatley, P., Navarro, M. et al. Synthesis of ‘unfeasible’ zeolites. Nature Chem 8, 58–62 (2016). https://doi.org/10.1038/nchem.2374
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2374
This article is cited by
-
Interchain-expanded extra-large-pore zeolites
Nature (2024)
-
Germanium-enriched double-four-membered-ring units inducing zeolite-confined subnanometric Pt clusters for efficient propane dehydrogenation
Nature Catalysis (2023)
-
Accurate large-scale simulations of siliceous zeolites by neural network potentials
npj Computational Materials (2022)
-
Microporous water with high gas solubilities
Nature (2022)
-
Theoretical design for zeolite synthesis
Science China Chemistry (2022)