Abstract
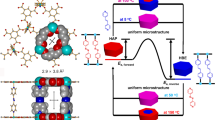
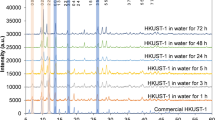
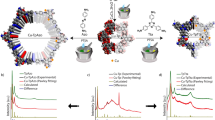
Post-synthetic metallation is employed strategically to imbue metal–organic frameworks (MOFs) with enhanced performance characteristics. However, obtaining precise structural information for metal-centred reactions that take place within the pores of these materials has remained an elusive goal, because of issues with high symmetry in certain MOFs, lower initial crystallinity for some chemically robust MOFs, and the reduction in crystallinity that can result from carrying out post-synthetic reactions on parent crystals. Here, we report a new three-dimensional MOF possessing pore cavities that are lined with vacant di-pyrazole groups poised for post-synthetic metallation. These metallations occur quantitatively without appreciable loss of crystallinity, thereby enabling examination of the products by single-crystal X-ray diffraction. To illustrate the potential of this platform to garner fundamental insight into metal-catalysed reactions in porous solids we use single-crystal X-ray diffraction studies to structurally elucidate the reaction products of consecutive oxidative addition and methyl migration steps that occur within the pores of the Rh-metallated MOF, 1·[Rh(CO)2][Rh(CO)2Cl2].
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ooi, L. Principles of X-ray Crystallography (Oxford Univ. Press, 2010).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Inokuma, Y. et al. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 495, 461–466 (2013); ibid. Corrigendum. Nature 501, 262 (2013).
Legrand, Y-M., van der Lee, A. & Barboiu, M. Single-crystal X-ray structure of 1,3-dimethylcyclobutadiene by confinement in a crystalline matrix. Science 329, 299–302 (2010).
Inokuma, Y., Kawano, M. & Fujita, M. Crystalline molecular flasks. Nature Chem. 3, 349–358 (2011).
Kawamichi, T., Haneda, T., Kawano, M. & Fujita, M. X-ray observation of a transient hemiaminal trapped in a porous network. Nature 461, 633–635 (2009).
Haneda, T., Kawano, M., Kawamichi, T. & Fujita, M. Direct observation of the labile imine formation through single-crystal-to-single-crystal reactions in the pores of a porous coordination network. J. Am. Chem. Soc. 130, 1578–1579 (2008).
Haneda, T., Kawano, M., Kojima, T. & Fujita, M. Thermo-to-photo switching of the chromic behavior of salicylideneanilines via inclusion in a porous coordination network. Angew. Chem. Int. Ed. 46, 6643–6645 (2007).
Kawano, M., Kobayashi, Y., Ozeki, T. & Fujita M. Direct crystallographic observation of a coordinatively unsaturated transition-metal complex in situ generated within a self-assembled cage. J. Am. Chem. Soc. 128, 6558–6559 (2006).
Kohyama, Y., Murase, T. & Fujita, M. Metal–organic proximity in a synthetic pocket. J. Am. Chem. Soc. 136, 2966–2969 (2014).
Zhang, J-P. & Chen, X-M. Optimised acetylene/carbon dioxide sorption in a dynamic porous crystal. J. Am. Chem. Soc. 131, 5516–5521 (2009).
Takamizawa, S. et al. Crystal transformation and host molecular motions in CO2 adsorption process of a metal benzoate pyrazine (MI=Rh, Cu). J. Am. Chem. Soc. 132, 3783–3792 (2010).
Vaidhyanathan, R. et al. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 330, 650–653 (2010).
Rowsell, J. L. C., Spenser, E. C., Eckert, J., Howard, J. A. K. & Yaghi. O. M. Gas adsorption sites in a large-pore metal–organic framework. Science 309, 1350–1354 (2005).
Vinogradova, E. V., Müller, P. & Buchwald, S. L. Structural reevaluation of the electrophilic hypervalent iodine reagent for trifluoromethylthiolation supported by the crystalline sponge method for X-ray analysis. Angew. Chem. Int. Ed. 126, 3189–3192 (2014).
Ikemoto, K., Inokuma, Y., Rissanen, K. & Fujita, M. X-ray snapshot observation of palladium-mediated aromatic bromination in a porous complex. J. Am. Chem. Soc. 136, 6892–6895 (2014).
Cohen, S. M. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 112, 970–1000 (2012).
Evans, J. D., Sumby, C. J. & Doonan, C. J. Post-synthetic metalation of metal–organic frameworks. Chem. Soc. Rev. 43, 5933–5951 (2014).
Bloch, E. D. et al. Metal insertion in a microporous metal organic framework lined with 2,2′-bipyridine. J. Am. Chem. Soc. 132, 14382–14384 (2010).
Bohnsack, A. M., Ibarra, I. A., Bakhmutov, V. I., Lynch, V. M. & Humphrey, S. M. Rational design of porous coordination polymers based on bis(phosphine)MCl2 complexes that exhibit high-temperature H2 sorption and chemical reactivity. J. Am. Chem. Soc. 135, 16038–16041 (2013).
Mulfort, K. L., Farha, O. K., Stern, C. L., Sarjeant, A. A. & Hupp, J. T. Post-synthesis alkoxide formation within metal–organic framework materials: a strategy for incorporating highly coordinatively unsaturated metal ions. J. Am. Chem. Soc. 131, 3866–3868 (2009).
Ma, L., Falkowski, J. M., Abney, C. & Lin, W. A series of isoreticular chiral metal–organic frameworks as a tunable platform for asymmetric catalysis. Nature Chem. 2, 838–846 (2010).
Canivet, J., Aguado, S., Schuurman, Y. & Farrusseng, D. MOF-supported selective ethylene dimerization single-site catalysts through one-pot postsynthetic modification. J. Am. Chem. Soc. 135, 4195–4198 (2013).
Wu, C-D., Hu A., Zhang L. & Lin W. A homochiral porous metal organic framework for highly enantioselective heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 127, 8940–8941 (2005).
Dau, P. V., Kim, M. & Cohen, S. M. Site-selective cyclometalation of a metal-organic framework. Chem. Sci. 4, 601–605 (2013).
Jacobs, T., Clowes, R., Cooper, A. I. & Hardie, M. J. A chiral, self-catenating and porous metal–organic framework and its post-synthetic metal uptake. Angew. Chem. Int. Ed. 51, 5192–5195 (2012).
Cotton, F. A. & Wilkinson, G. Advanced Inorganic Chemistry (Wiley, 1988).
Bloch, W. M. & Sumby, C. J. Guest-induced crystal-to-crystal expansion and contraction of a 3-D porous coordination polymer. Chem. Commun. 48, 2534–2536 (2012).
Bloch, W. M., Babarao, R., Hill, M. R., Doonan, C. J. & Sumby, C. J. Post-synthetic structural processing in a metal–organic framework material as a mechanism for exceptional CO2/N2 selectivity. J. Am. Chem. Soc. 135, 10441–10448 (2013).
Bloch, W. M., Doonan, C. J. & Sumby, C. J. Using hinged ligands to target structurally flexible copper(II) MOFs. CrystEngComm 15, 9663–9671 (2013).
Keene, T. D. et al. Solvent-modified dynamic porosity in chiral 3D kagome frameworks. Dalton Trans. 42, 7871–7879 (2013).
Pettinari, C., Santini, C., Leonesi, D., Cecchi, P. Synthesis and characterization of some zinc, cadmium and mercury(II) derivatives of bis(4-methylpyrazol-1-yl) alkanes. Polyhedron 13, 1553–1562 (1994).
Trofimenko, S. Coordination chemistry of pyrazole-derived ligands. Chem. Rev. 72, 497–509 (1972).
Shultz, A. M., Sarjeant, A. A., Farha, O. K., Hupp, J. T. & Nguyen, S. T. Post-synthesis modification of a metal–organic framework to form metallosalen-containing MOF materials. J. Am. Chem. Soc. 133, 13252–13255 (2011).
Morris, W. et al. Synthesis, structure, and metalation of two new highly porous zirconium metal–organic frameworks. Inorg. Chem. 51, 6443–6445 (2012).
Teuma, E. et al. Tandem carbonylation reactions: hydroformylation and hydroaminomethylation of alkenes catalyzed by cationic [(H2C(3,5-Me2pz)2)Rh(CO)L]+ complexes. Organometallics 22, 5261–5267 (2003).
Oro, L. A. et al. Rhodium(I) complexes with bis(pyrazolyl)methane crystal structure of [Rh(COD)(CH2(Pz)2)]ClO4·½ C2H4Cl2 . J. Organomet. Chem. 276, 79–97 (1984).
Ellis, P. R. et al. Oxidative addition of alkyl halides to rhodium(I) and iridium(I) dicarbonyl diiodides: key reactions in the catalytic carbonylation of alcohols. Organometallics 13, 3215–3226 (1994).
Nguyen, D. H. et al. Reductive elimination of anhydrides from anionic iodo acetyl carboxylato rhodium complexes. Eur. J. Inorg. Chem. 2014, 326–336 (2014).
Conifer, C. M. et al. Dicarbonylrhodium(I) complexes of bipyridine ligands with proximate H-bonding substituents and their application in methyl acetate carbonylation. Eur. J. Inorg. Chem. 2011, 3511–3522 (2011).
Montag, M. et al. CO-induced methyl migration in a rhodium thiophosphoryl pincer complex and its comparison with phosphine-based complexes: the divergent effects of S and P donor ligands. Organometallics 32, 7163–7180 (2013).
Peverati, R. & Truhlar, D. G. M11-L: a local density functional that provides improved accuracy for electronic structure calculations in chemistry and physics. J. Phys. Chem. Lett. 3, 117–124 (2012).
Lee, K. et al. Design of a metal–organic framework with enhanced back bonding for separation of N2 and CH4 . J. Am. Chem. Soc. 136, 698–704 (2013).
Feliz, M., Freixa, Z., van Leeuwen, P. W. N. M. & Bo, C. Revisiting the methyl iodide oxidative addition to rhodium complexes: a DFT study of the activation parameters. Organometallics 24, 5718–5723 (2005).
Ingleson, M. J., Barrio, J. P., Guilbaud, J-P., Khimyak, Y. Z. & Rosseinsky, M. J. Framework functionalisation triggers metal binding. Chem. Commun. 2680–2682 (2008).
Sheldrick, G. M. Phase annealing in SHELX-90: direct methods for larger structures. Acta Crystallogr. A 46, 467–473 (1990).
Sheldrick, G. M. SHELXL-97 (University of Göttingen, 1997).
Barbour, L. J. A software tool for supramolecular crystallography. J. Supramol. Chem. 1, 189–191 (2001).
Acknowledgements
C.J.D. and C.J.S. acknowledge the Science and Industry Endowment Fund for financial support and the Australian Research Council for funding Future Fellowships (FT100100400 and FT0991910). Aspects of this research were undertaken on the MX1 and MX2 beamlines at the Australian Synchrotron, Victoria, Australia. M.L.C. acknowledges generous allocations of supercomputing time on the National Facility of the National Computational Infrastructure and an ARC Future Fellowship (FT100100320). Correspondence and requests for materials should be addressed to C.J.D. and C.J.S.
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper. W.M.B. and A.B. undertook most of the synthetic work and analysis. C.J.C. measured and interpreted the ICP-MS data. W.M.B., A.B., C.J.C. and C.J.S. collected, solved and refined the SCXRD data. R.L. and M.L.C. undertook the computational studies. W.M.B., C.J.D. and C.J.S. wrote the manuscript with input from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2999 kb)
Supplementary information
Crystallographic data for compound 1-des (CIF 32 kb)
Supplementary information
Crystallographic data for compound 1.[Co(H2O)4]Cl2 (CIF 6735 kb)
Supplementary information
Crystallographic data for compound 1.[CoCl2] (CIF 32894 kb)
Supplementary information
Crystallographic data for compound 1.[Rh(CO)2][RhCl2(CO)2] (CIF 36 kb)
Supplementary information
Crystallographic data for compound 1.[Rh(H2O)4]Cl3 (CIF 35 kb)
Supplementary information
Crystallographic data for compound 1.[Rh(COMe)(CO)(CH3CN)I]I (CIF 4420 kb)
Supplementary information
Crystallographic data for compound 1.DMF (CIF 53 kb)
Rights and permissions
About this article
Cite this article
Bloch, W., Burgun, A., Coghlan, C. et al. Capturing snapshots of post-synthetic metallation chemistry in metal–organic frameworks. Nature Chem 6, 906–912 (2014). https://doi.org/10.1038/nchem.2045
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2045
This article is cited by
-
Metal-organic framework boosts heterogeneous electron donor–acceptor catalysis
Nature Communications (2023)
-
Symmetry-breaking dynamics in a tautomeric 3D covalent organic framework
Nature Communications (2023)
-
A spin-crossover framework endowed with pore-adjustable behavior by slow structural dynamics
Nature Communications (2022)
-
Chelating Metal Ions in a Metal-Organic Framework for Constructing a Biomimetic Catalyst Through Post-modification
Chemical Research in Chinese Universities (2022)
-
Giant single-crystal-to-single-crystal transformations associated with chiral interconversion induced by elimination of chelating ligands
Nature Communications (2021)