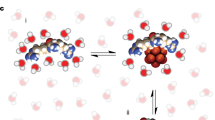
Abstract
Anion transporters based on small molecules have received attention as therapeutic agents because of their potential to disrupt cellular ion homeostasis. However, a direct correlation between a change in cellular chloride anion concentration and cytotoxicity has not been established for synthetic ion carriers. Here we show that two pyridine diamide-strapped calix[4]pyrroles induce coupled chloride anion and sodium cation transport in both liposomal models and cells, and promote cell death by increasing intracellular chloride and sodium ion concentrations. Removing either ion from the extracellular media or blocking natural sodium channels with amiloride prevents this effect. Cell experiments show that the ion transporters induce the sodium chloride influx, which leads to an increased concentration of reactive oxygen species, release of cytochrome c from the mitochondria and apoptosis via caspase activation. However, they do not activate the caspase-independent apoptotic pathway associated with the apoptosis-inducing factor. Ion transporters, therefore, represent an attractive approach for regulating cellular processes that are normally controlled tightly by homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 September 2014
The authors wish to add a sentence to the Acknowledgements: "A part of this work was carried out with support from the Chemical Biology Research Center in Korea Research Institute of Bioscience and Biotechnology." This has been added in all versions of the Article.
References
Yu, S. P., Canzoniero, L. M. T. & Choi, D. W. Ion homeostasis and apoptosis. Curr. Opin. Cell Biol. 13, 405–411 (2001).
Okada, Y. et al. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 209, 21–29 (2006).
Newmeyer, D. D. & Ferguson-Miller, S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112, 481–490 (2003).
Arcangeli, A. et al. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr. Med. Chem. 16, 66–93 (2009).
Lehen'kyi, V., Shapovalov, G., Skryma, R. & Prevarskaya, N. Ion channels and transporters in cancer. 5. Ion channels in control of cancer and cell apoptosis. Am. J. Physiol. Cell Physiol. 301, C1281–C1289 (2011).
Becchetti, A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am. J. Physiol. Cell Physiol. 301, C255–C265 (2011).
Cuddapah, V. A. & Sontheimer, H. Ion channels and transporters in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 301, C541–C549 (2011).
Yu, L. et al. A protective mechanism against antibiotic-induced ototoxicity: role of prestin. PloS ONE 6, e17322 (2011).
Tsukimoto, M., Harada, H., Ikari, A. & Takagi, K. Involvement of chloride in apoptotic cell death induced by activation of ATP-sensitive P2X7 purinoceptor. J. Biol. Chem. 280, 2653–2658 (2005).
Lee, J. M., Davis, F. M., Roberts-Thomson S. J. & Monteith, G. R. Ion channels and transporters in cancer. 4. Remodeling of Ca2+ signaling in tumorigenesis: role of Ca2+ transport. Am. J. Physiol. Cell Physiol. 301, C969–C976 (2011).
Remillard, C. V. & Yuan, J. X-J. Activation of K+ channels: an essential pathway in programmed cell death. Am. J. Physiol. Lung Cell Mol. Physiol. 286, L49–L67 (2004).
Gupta, P. B. et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–659 (2009).
Ding, W-Q., Liu, B., Vaught, J. L., Yamauchi, H. & Lind, S. E. Anticancer activity of the antibiotic clioquinol. Cancer Res. 65, 3389–3395 (2005).
Fürstner, A. Chemistry and biology of roseophilin and the prodigiosin alkaloids: a survey of the last 2500 years. Angew. Chem. Int. Ed. 42, 3582–3603 (2003).
Busschaert, N. et al. Structure–activity relationships in tripodal transmembrane anion transporters: the effect of fluorination. J. Am. Chem. Soc. 133, 14136–14148 (2011).
Busschaert, N. et al. Towards predictable transmembrane transport: QSAR analysis of anion binding and transport. Chem. Sci. 4, 3036–3045 (2013).
Shen, B., Li, X., Wang, F., Yao, X. & Yang, D. A synthetic chloride channel restores chloride conductance in human cystic fibrosis epithelial cells. PLoS ONE 7, e34694 (2012).
Sato, T. et al. Prodigiosins as a new group of H+/Cl− symporters that uncouple proton translocators. J. Biol. Chem. 273, 21455–21462 (1998).
Sessler, J. L. et al. Synthesis, anion-binding properties, and in vitro anticancer activity of prodigiosin analogues. Angew. Chem. Int. Ed. 44, 5989–5992 (2005).
Gale, P. A. et al. Co-transport of H+/Cl− by a synthetic prodigiosin mimic. Chem. Commun. 30, 3773–3775 (2005).
Pérez-Tomás, R., Montaner, B., Llagostera, E. & Soto-Cerrato, V. The prodigiosins, proapoptotic drugs with anticancer properties. Biochem. Pharmacol. 66, 1447–1452 (2003).
Melvin, M. S. et al. Double-strand DNA cleavage by copper prodigiosin. J. Am. Chem. Soc. 122, 6333–6334 (2000).
Soto-Cerrato, V., Viñals, F., Lambert, J. R. & Pérez-Tomás, R. The anticancer agent prodigiosin induces 21(WAF1/C1P1) expression via transforming growth factor-beta receptor pathway. Biochem. Pharmacol. 74, 1340–1349 (2007).
Davis, A. P., Sheppard, D. N. & Smith, B. D. Development of synthetic membrane transporters for anions. Chem. Soc. Rev. 36, 348–357 (2007).
Davis, J. T., Okunola, O. & Quesada, R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 39, 3843–3862 (2010).
Gale, P. A. From anion receptors to transporters. Acc. Chem. Res. 44, 216–226 (2011).
Díaz de Greñu, B. et al. Synthetic prodiginine obatoclax (GX15-070) and related analogues: anion binding, transmembrane transport, and cytotoxicity properties. Chem. Eur. J. 17, 14074–14083 (2011).
Moore, S. J. et al. Towards ‘drug-like’ indole-based transmembrane anion transporters. Chem. Sci. 3, 2501–2509 (2012).
Li, X., Shen, B., Yao, X-Q. & Yang, D. Synthetic chloride channel regulates cell membrane potentials and voltage-gated calcium channels. J. Am. Chem. Soc. 131, 13676–13680 (2009).
Gould, D. Human physiology. From cells to systems, 3rd edition. J. Adv. Nurs. 28, 680–682 (1998).
Russell, J. M. Sodium–potassium–chloride cotransport. Physiol. Rev. 80, 211–276 (2000).
Moore, S. J., Fisher, M. G., Yano, M., Tong, C. C. & Gale, P. A. A dual host approach to transmembrane transport of salts. Chem. Commun. 47, 689–691 (2011).
Yoon, D-W., Hwang, H. & Lee, C-H. Synthesis of a strapped calix[4]pyrrole: structure and anion binding properties. Angew. Chem. Int. Ed. 41, 1757–1759 (2002).
Lee, C-H., Miyaji, H., Yoon, D-W. & Sessler, J. L. Strapped and other topographically nonplanar calixpyrrole analogues. Improved anion receptors. Chem. Commun. 24–34 (2008).
Custelcean, R. et al. Calix[4]pyrrole: an old yet new ion-pair receptor. Angew. Chem. Int. Ed. 44, 2537–2542 (2005).
Delort, A-M., Gaudet, G. & Forano E. Environmental Microbiology: Methods and Protocols (eds Spencer, J. F. T. & Ragout de Spencer, A. L.) 389–405 (Humana Press, 2004).
Jayaraman, S., Haggie, P., Wachter, R. M., Remington, S. J. & Verkman, A. S. Mechanism and cellular applications of a green fluorescent protein-based halide sensor. J. Biol. Chem. 275, 6047–6050 (2000).
Minta A. & Tsien R. Y. Fluorescent indicators for cytosolic sodium. J. Biol. Chem. 264, 19449–19457 (1989).
Meuwis, K., Boens, N., De Schryver, F. C., Gallay, J. & Vincent, M. Photophysics of the fluorescent K+ indicator PBFI. Biophys. J. 68, 2469–2473 (1995).
Gee, K. R. et al. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium 27, 97–106 (2000).
Williams, D. R., Ko, S-K., Park, S., Lee, M-R. & Shin, I. An apoptosis-inducing small molecule that binds to heat shock protein 70. Angew. Chem. Int. Ed. 47, 7466–7469 (2008).
Salvioli, S., Ardizzoni, A., Franceschi, C. & Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 411, 77–82 (1997).
Elmore, S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 25, 495–516 (2007).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Joza, N. et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410, 549–554 (2001).
Bortner, C. D. & Cidlowski, J. A. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J. Biol. Chem. 278, 39176–39184 (2003).
Ko, S-K. & Shin, I. Cardiosulfa induces heart deformation in zebrafish through the AhR-mediated, CYP1A-independent pathway. ChemBioChem 13, 1483–1489 (2012).
Han, J. & Burgess, K. Fluorescent indicators for intracellular pH. Chem. Rev. 110, 2709–2728 (2010).
Cho, H. J. et al. A small molecule that binds to an ATPase domain of Hsc70 promotes membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator. J. Am. Chem. Soc. 133, 20267–20276 (2011).
Circu, M. L. & Aw, T. Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48, 749–762 (2010).
Acknowledgements
This work was supported by the National Creative Research Initiative (grant no. 2010-0018272 to I.S.) program in Korea, as well as by the Office of Basic Energy Sciences, US Department of Energy (grant no. DE-FG02-01ER15186 to J.L.S.). P.A.G. thanks the Engineering and Physical Sciences Research Council for a postdoctoral fellowship (N.B.) (EP/J009687/1). W.VR. and P.A.G. thank the European Union for a Marie Curie Career Integration grant. A part of this work was carried out with support from the Chemical Biology Research Center in Korea Research Institute of Bioscience and Biotechnology.
Author information
Authors and Affiliations
Contributions
J.L.S, I.S. and P.A.G. designed the study and supervised the work. S-K.K. performed biological studies. S.K.K. designed and synthesized compounds and performed ion-binding studies in solution. S.K.K. and V.M.L. carried out the X-ray single-crystal structure analysis. P.A.G., W.VR., N.B. and A.S. designed and performed the ion-transport studies in liposomes. J.P. and W.N. carried out ion-transport activity studies in cells.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 10471 kb)
Supplementary information
Crystallographic data for 1·CsCl (CIF 1298 kb)
Supplementary information
Crystallographic data for 12·NaCl·TMACl·(CH3OH)2·(H2O)2 (CIF 1601 kb)
Supplementary information
Crystallographic data for 2·CsCl (CIF 1206 kb)
Supplementary information
Crystallographic data for 22·NaCl·TMACl·(H2O)5·C6H14 (CIF 2987 kb)
Supplementary information
Crystallographic data for 2·TEACl (CIF 39 kb)
Supplementary information
Crystallographic data for 1·(CH3OH)2 (CIF 29 kb)
Supplementary information
Crystallographic data for 3·(CH3OH)2 (CIF 29 kb)
Rights and permissions
About this article
Cite this article
Ko, SK., Kim, S., Share, A. et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nature Chem 6, 885–892 (2014). https://doi.org/10.1038/nchem.2021
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2021
This article is cited by
-
In vivo therapy of osteosarcoma using anion transporters-based supramolecular drugs
Journal of Nanobiotechnology (2024)
-
Molecular rotaxane shuttle-relay accelerates K+/Cl− symport across a lipid membrane
Science China Chemistry (2023)
-
Aryl- and Superaryl-Extended Calix[4]pyrroles: From Syntheses to Potential Applications
Topics in Current Chemistry (2023)
-
Measuring anion binding at biomembrane interfaces
Nature Communications (2022)
-
Two calix[4]pyrroles as potential therapeutics for castration-resistant prostate cancer
Investigational New Drugs (2022)