Abstract
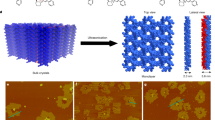
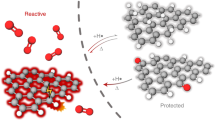
The rise of graphene, a natural two-dimensional polymer (2DP) with topologically planar repeat units, has challenged synthetic chemistry, and has highlighted that accessing equivalent covalently bonded sheet-like macromolecules has, until recently, not been achieved. Here we show that non-centrosymmetric, enantiomorphic single crystals of a simple-to-make monomer can be photochemically converted into chiral 2DP crystals and cleanly reversed back to the monomer. X-ray diffraction established unequivocal structural proof for this synthetic 2DP, which has an all-carbon scaffold and can be synthesized on the gram scale. The monomer crystals are highly robust, can be easily grown to sizes greater than 1 mm and the resulting 2DP crystals exfoliated into nanometre-thin sheets. This unique combination of features suggests that these 2DPs could find use in membranes and nonlinear optics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schmidt, G. M. J. Photodimerization in the solid state. Pure Appl. Chem. 12, 647–678 (1971).
Wegner, G. Topochemical reactions of monomers with conjugated triple bonds. I. Polymerization of 2,4-hexadiyn-1,6-diol derivatives in crystalline state. Z. Naturforsch. 24B, 824–832 (1969).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nature Chem. 4, 287–291 (2012).
Bhola, R. et al. A two-dimensional polymer from the anthracene dimer and triptycene motifs. J. Am. Chem. Soc. 135, 14134–14140 (2013).
Sakamoto, J., van Heijst, J., Lukin, O. & Schlüter, A. D. Two-dimensional polymers: just a dream of synthetic chemists? Angew. Chem. Int. Ed. 48, 1030–1069 (2009).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nature Chem. 5, 453–465 (2013).
Staudinger H. Concerning polymerization. Ber. Dtsch. Chem. Ges. 53, 1073–1085 (1920).
Berlanga, I. et al. Delamination of layered covalent organic frameworks. Small 7, 1207–1211 (2011).
Spitler, E. L. et al. A 2D covalent organic framework with 4.7-nm pores and insight into its interlayer stacking. J. Am. Chem. Soc. 133, 19416–19421(2011).
Amo-Ochoa, P. et al. Single layers of a multifunctional laminar Cu(I,II) coordination polymer. Chem. Commun. 46, 3262–3264 (2010).
Li, P., Maeda, Y. & Xu, Q. Top-down fabrication of crystalline metal–organic framework nanosheets. Chem. Commun. 47, 8436–8438 (2011).
Gallego, A. et al. Solvent-induced delamination of a multifunctional two dimensional coordination polymer. Adv. Mater. 25, 2141–2146 (2013).
Bauer, T. et al. Synthesis of free-standing, monolayered organometallic sheets at the air/water interface. Angew. Chem. Int. Ed. 50, 7879–7884 (2011).
Zheng, Z. et al. Square-micrometer-sized, free-standing organometallic sheets and their square-centimeter-sized multilayers on solid substrates. Macromol. Rapid Commun. 34, 1670–1680 (2013).
Payamyar, P. et al. Synthesis of a covalent monolayer sheet by photochemical anthracene dimerization at the air/water interface and its mechanical characterization by AFM indentation. Adv. Mater. 26, 2052–2058 (2014).
Bunck, D. N. & Dichtel, W. R. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 135, 14952–14955 (2013).
Colson, J. W. et al. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 332, 228–231 (2011).
Bianco, E. et al. Stability and exfoliation of germanane: a germanium graphane analogue. ACS Nano 7, 4414–4421 (2013).
Kuhn, A., Holzmann, T., Nuss, J. & Lotsch, B. V. A facile wet chemistry approach towards unilamellar tin sulfide nanosheets from Li4 xSn1– xS2 solid solutions. J. Mater. Chem. A 2, 6100–6106 (2014).
Xu, M., Liang, T., Shi, M. & Chen, H. Graphene-like two dimensional materials. Chem. Rev. 113, 3766–3798 (2013).
Beaudoin, D., Maris, T. & Wuest, J. D. Constructing monocrystalline covalent organic networks by polymerization. Nature Chem. 5, 830–834 (2013).
Côte, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Uribe-Romo, F. J. et al. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 133, 11478–11481 (2011).
Kuhn, P., Antonietti, M. & Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47, 3450–3453 (2008).
Kory, M. J., Bergeler, M., Reiher, M. & Schlüter, A. D. Facile synthesis and theoretical conformation analysis of a triazine-based double-decker rotor molecule with three anthracene blades. Chem. Eur. J. 20, 6934–6938 (2014).
Thomas, J. M. Organic reactions in the solid state: accident and design. Pure Appl. Chem. 51, 1965–1082 (1979).
Hernandez, Y. et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nature Nanotech. 3, 563–568 (2008).
Ernst, K. H. Molecular chirality in surface science. Surf. Sci. 613, 1–5 (2013).
Bouas-Laurent, H., Castellan, A., Desvergne, J. P. & Lapouyade, R. Photodimerization of anthracenes in fluid solutions: (part 2) mechanistic aspects of the photocycloaddition and of the photochemical and thermal cleavage. Chem. Soc. Rev. 30, 248–263 (2001).
Tomlinson, W. J. et al. Reversible photodimerization: a new type of photochromism. Appl. Optics 11, 533–548 (1972).
Acknowledgements
T. Schweizer is thanked for building the ultraviolet reactor and the PID (proportional integral derivative)-controlled heating plate. We thank R. Verel for help with the acquisition of solid-state NMR spectra and G. Wegner, B. King, B. Batlogg, J. Leuthold and W. Steurer for helpful discussions. We further thank F. Heiligtag for the help with the acquisition of solid-state ultraviolet absorbance and fluorescence spectra and A. Reiser for assisting with AFM image acquisition and processing. P. Smith and K. Feldmann are thanked for the access to optical microscopy.
Author information
Authors and Affiliations
Contributions
M.J.K. designed and performed most of the experiments. T.W., M.W. and N.T. carried out the X-ray crystal structure measurements, and analysed and interpreted the data. P.P. performed the AFM height analyses and helped with the acquisition of SEM images. S.W.v.d.P. carried out some of the exfoliation experiments under the supervision of M.J.K. J.D. performed the X-ray powder diffraction measurements and created the X-ray structure illustrations presented in this paper. A.D.S. initiated the activities for 2DP synthesis, designed the monomer and coordinated the research. A.D.S and M.J.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5858 kb)
Supplementary information
Crystallographic data for compound 1 (CIF 1670 kb)
Supplementary information
Crystallographic data for compound 1-partially polymerized (CIF 34 kb)
Supplementary information
Crystallographic data for compound 1-polymer (CIF 673 kb)
Supplementary information
Crystallographic data for compound 1-polymer-annealed (CIF 4147 kb)
Supplementary information
Crystallographic data for compound 1_depolymerized (CIF 34 kb)
Rights and permissions
About this article
Cite this article
Kory, M., Wörle, M., Weber, T. et al. Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction. Nature Chem 6, 779–784 (2014). https://doi.org/10.1038/nchem.2007
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2007
This article is cited by
-
Irreversible synthesis of an ultrastrong two-dimensional polymeric material
Nature (2022)
-
Reconstructed covalent organic frameworks
Nature (2022)
-
Molecular soccer balls connected to make a 2D material
Nature (2022)
-
Free-standing homochiral 2D monolayers by exfoliation of molecular crystals
Nature (2022)
-
Observing polymerization in 2D dynamic covalent polymers
Nature (2022)