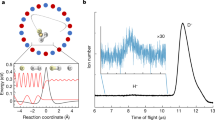
Abstract
Quantum phenomena in the translational motion of reactants, which are usually negligible at room temperature, can dominate reaction dynamics at low temperatures. In such cold conditions, even the weak centrifugal force is enough to create a potential barrier that keeps reactants separated. However, reactions may still proceed through tunnelling because, at low temperatures, wave-like properties become important. At certain de Broglie wavelengths, the colliding particles can become trapped in long-lived metastable scattering states, leading to sharp increases in the total reaction rate. Here, we show that these metastable states are responsible for a dramatic, order-of-magnitude-strong, quantum kinetic isotope effect by measuring the absolute Penning ionization reaction rates between hydrogen isotopologues and metastable helium down to 0.01 K. We demonstrate that measurements of a single isotope are insufficient to constrain ab initio calculations, making the kinetic isotope effect in the cold regime necessary to remove ambiguity among possible potential energy surfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Urey, H., Brickwedde, F. & Murphy, G. A hydrogen isotope of mass 2. Phys. Rev. 39, 164–165 (1932).
Urey, H., Brickwedde, F. & Murphy, G. A hydrogen isotope of mass 2 and its concentration. Phys. Rev. 40, 1–15 (1932).
Washburn, E. W. & Urey, H. C. Concentration of the H2 isotope of hydrogen by the fractional electrolysis of water. Proc. Natl Acad. Sci. USA 18, 496–498 (1932).
Westheimer, F. H. The magnitude of the primary kinetic isotope effect for compounds of hydrogen and deuterium. Chem. Rev. 61, 265–273 (1961).
Ospelkaus, S. et al. Quantum-state controlled chemical reactions of ultracold potassium–rubidium molecules. Science 327, 853–857 (2010).
Henson, A. B., Gersten, S., Shagam, Y., Narevicius, J. & Narevicius, E. Observation of resonances in Penning ionization reactions at sub-kelvin temperatures in merged beams. Science 338, 234–238 (2012).
Ni, K-K. et al. Dipolar collisions of polar molecules in the quantum regime. Nature 464, 1324–1328 (2010).
Chefdeville, S. et al. Observation of partial wave resonances in low-energy O2–H2 inelastic collisions. Science 341, 1094–1096 (2013).
Millar, T. J. Deuterium fractionation in interstellar clouds. Space Sci. Rev. 106, 73–86 (2003).
Millar, T. J., Bennett, A. & Herbst, E. Deuterium fractionation in dense interstellar clouds. Astrophys. J. 340, 906 (1989).
Messenger, S. Identification of molecular-cloud material in interplanetary dust particles. Nature 404, 968–971 (2000).
Bell, M. T., Bell, P. & Softley, T. Ultracold molecules and ultracold chemistry. Mol. Phys. 107, 99–132 (2009).
Wigner, E. On the behavior of cross sections near thresholds. Phys. Rev. 73, 1002–1009 (1948).
Krems, R. V. Molecules near absolute zero and external field control of atomic and molecular dynamics. Int. Rev. Phys. Chem. 24, 99–118 (2005).
Herschbach, D. Molecular collisions, from warm to ultracold. Faraday Discuss. 142, 9 (2009).
Skodje, R. T. et al. Observation of a transition state resonance in the integral cross section of the F + HD reaction. J. Chem. Phys. 112, 4536 (2000).
Skodje, R. et al. Resonance-mediated chemical reaction: F + HD→HF + D. Phys. Rev. Lett. 85, 1206–1209 (2000).
Qiu, M. et al. Observation of Feshbach resonances in the F + H2→HF + H reaction. Science 311, 1440–1443 (2006).
Ren, Z. et al. Probing the resonance potential in the F atom reaction with hydrogen deuteride with spectroscopic accuracy. Proc. Natl Acad. Sci. USA 105, 12662–12666 (2008).
Kirste, M. et al. Quantum-state resolved bimolecular collisions of velocity-controlled OH with NO radicals. Science 338, 1060–1063 (2012).
Janssen, L. M. C., van der Avoird, A. & Groenenboom, G. C. Quantum reactive scattering of ultracold NH(X3Σ−) radicals in a magnetic trap. Phys. Rev. Lett. 110, 063201 (2013).
Janssen, L. M. C., Żuchowski, P. S., van der Avoird, A., Hutson, J. M. & Groenenboom, G. C. Cold and ultracold NH–NH collisions: the field-free case. J. Chem. Phys. 134, 124309 (2011).
Druyvesteyn, M. & Penning, F. The mechanism of electrical discharges in gases of low pressure. Rev. Mod. Phys. 12, 87–174 (1940).
Wiley, W. C. & McLaren, I. H. Time-of-flight mass spectrometer with improved resolution. Rev. Sci. Instrum. 26, 1150 (1955).
Siska, P. E. Molecular-beam studies of Penning ionization. Rev. Mod. Phys. 65, 337–412 (1993).
Hapka, M., Chałasiński, G., Kłos, J. & Żuchowski, P. S. First-principle interaction potentials for metastable He(3S) and Ne(3P) with closed-shell molecules: application to Penning-ionizing systems. J. Chem. Phys. 139, 014307 (2013).
Żuchowski, P. & Hutson, J. Low-energy collisions of NH3 and ND3 with ultracold Rb atoms. Phys. Rev. A 79, 062708 (2009).
Janssen, L. M. C., Żuchowski, P. S., van der Avoird, A., Groenenboom, G. C. & Hutson, J. M. Cold and ultracold NH–NH collisions in magnetic fields. Phys. Rev. A 83, 022713 (2011).
Even, U., Jortner, J., Noy, D., Lavie, N. & Cossart-Magos, C. Cooling of large molecules below 1 K and He clusters formation. J. Chem. Phys. 112, 8068 (2000).
Luria, K., Lavie, N. & Even, U. Dielectric barrier discharge source for supersonic beams. Rev. Sci. Instrum. 80, 104102 (2009).
Shagam, Y. & Narevicius, E. Sub-kelvin collision temperatures in merged neutral beams by correlation in phase-space. J. Phys. Chem. C 117, 22454–22461 (2013).
Chefdeville, S. et al. Appearance of low energy resonances in CO–para-H2 inelastic collisions. Phys. Rev. Lett. 109, 1–5 (2012).
Acknowledgements
The authors thank U. Even and Y. Prior for discussions. The authors thank J.W. Rosenberg for reading the manuscript. This research was made possible, in part, by the historic generosity of the Harold Perlman family. E.N. acknowledges support from the Israel Science Foundation and the Minerva Foundation. J.K. acknowledges financial support through the United States National Science Foundation (grant no. CHE-1213332) to M. Alexander. P.S.Z. was supported by the Iuventus Plus grant by the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Contributions
The experimental work and data analysis were carried out by E.L-O., Y.S., A.B.H., S.G., J.N. and E.N. The ab initio potential surfaces were calculated by J.K. and P.S.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 302 kb)
Rights and permissions
About this article
Cite this article
Lavert-Ofir, E., Shagam, Y., Henson, A. et al. Observation of the isotope effect in sub-kelvin reactions. Nature Chem 6, 332–335 (2014). https://doi.org/10.1038/nchem.1857
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1857
This article is cited by
-
Anisotropic dynamics of resonant scattering between a pair of cold aligned diatoms
Nature Chemistry (2022)
-
Mapping partial wave dynamics in scattering resonances by rotational de-excitation collisions
Nature Chemistry (2022)
-
Determining the nature of quantum resonances by probing elastic and reactive scattering in cold collisions
Nature Chemistry (2021)
-
Towards chemistry at absolute zero
Nature Reviews Chemistry (2021)
-
Selectivity of weak intermolecular forces and precursor state of elementary oxidation reactions, a new insight on Ne* + N2 chemiionization
Scientific Reports (2021)