Abstract
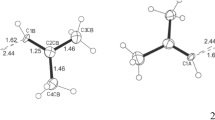
Compounds that deviate from the normal range of bonding can help to assess the strengths and weaknesses of the models currently used to describe chemical bonds. Furthermore, computer simulations of molecules require experimental data to describe accurately the energies and forces between interacting molecules. Compounds that contain the trinitromethyl group, with three nitro groups bonded to one carbon atom, show remarkable inter- and intramolecular effects. In this paper, we report the structural features of chlorotrinitromethane in the solid state and present the first reliable solid-state geometry parameters of an α-halogen derivative of the trinitromethyl pseudohalogen. We found several intriguing geometrical features in terms of intra- and intermolecular interactions, as well as an exceptionally short carbon–chlorine bond (1.694(1) Å). Using a combined crystallographic and computational approach, we show that these effects can be described in terms of the computed electrostatic potential of the molecular surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hoffmann, R. & Hopf, H. Learning from molecules in distress. Angew. Chem. Int. Ed. 47, 4474–4481 (2008).
Stone, A. J. Intermolecular potentials. Science 321, 787–789 (2008).
Metrangolo, P., Meyer, F., Pilati, T., Resnati, G. & Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 47, 6114–6127 (2008).
Metrangolo, P. & Resnati, G. Halogen versus hydrogen. Science 321, 918–919 (2008).
Göbel, M. & Klapötke, T. M. Development and testing of energetic materials: the concept of high densities based on the trinitroethyl functionality. Adv. Funct. Mater. 19, 347–365 (2009).
Birckenbach, L., Huttner, K. & Stein, W. Die Hydrolysen-Konstanten des Brom-tricyanmethyls und des Chlor-, Brom- und Jod-trinitromethyls. Chem. Ber. 62, 2065–2075 (1929).
Pepkin, V. I., Matyushin, Yu. N., Khisamutdinov, G. Kh., Slovetskii, V. I. & Fainzil'berg, A. A. Thermochemical properties of α-azidopolynitroalkanes and the dissociation energy of C–N3 bonds in organic azides. Khim. Fiz. 12, 1399–1403 (1993).
Khisamutdinov, G. Kh., Slovetskii, V. I., Golub, M. Yu., Shevelev, S. A. & Fainzil'berg, A. A. α-Azidopolynitroalkanes. Synthesis and vibrational spectra. Russ. Chem. Bull. 46, 324–327 (1997).
Parker, C. O., Emmons, W. E., Rolewicz, H. A. & McCallum, K. S. Chemistry of dinitroacetonitrile – I: Preparation and properties of dinitroacetonitrile and its salts. Tetrahedron 17, 79–87 (1962).
Macbeth, A. K. & Pratt, D. D. XLIII. – The halogen derivatives of nitroform. J. Chem. Soc. 119, 354–358 (1921).
Will, W. Hexanitroethane. Chem. Ber. 47, 961–965 (1914).
Levchenkov, D. V., Kharitonkin, A. B. & Shlyapochnikov, V. A. Molecular structures of trinitromethane derivatives. Russ. Chem. Bull. 50, 385–389 (2001).
Shlyapochnikov, V. A., Levchenkov, D. V. & Kharitonkin, A. B. Vibrational spectra of trinitromethane derivatives. Russ. Chem. Bull. 50, 1173–1180 (2001).
Vulfson, S. G., Timosheva, A. P., Vereshchagin, A. N. & Arbuzov, B. A. Molecular polarizability and the three-dimensional structure of substituted trinitromethanes. J. Mol. Struct. 40, 225–231 (1977).
Shlyapochnikov, V. A., Fainzil'berg, A. A. & Novikov, S. S. The spectra and structures of halogen derivatives of trinitromethane. Izv. Akad. Nauk SSSR 3, 519–520 (1961).
Sadova, N. I., Popik, N. I., Vilkov, L. V., Pankrushev, Ju. A. & Shlyapochnikov, V. A. Electron diffraction study of gaseous CH(NO2)3 and CCl(NO2)3 . J. Chem. Soc. Chem. Commun. 19, 708–709 (1973).
Sadova, N. I., Popik, N. I. & Vilkov, L. V. Electron–diffraction investigation of the structure of the HC(NO2)3, ClC(NO2)3, and BrC(NO2)3 molecules in the gas phase. Zh. Strukt Khim. 17, 298–303 (1976).
Demaison, J., Wlodarczak, G. & Rudolph, H. D. in Advances in Molecular Structure Research, Vol. 3 (eds Hargittai, I. & Hargittai, M.) 1–51 (JAI Press: Greenwich, 1997).
Demaison, J., Margulès, L & Boggs, J. E. The equilibrium C–Cl, C–Br, and C–I bond lengths from ab initio calculations, microwave and infrared spectroscopies, and empirical correlations. Struct. Chem. 14, 159–174 (2003).
Golovina, N. I. & Atovmyan, L. O. Crystal structure of iodotrinitromethane. Zh. Strukt. Khim. 7, 235–239 (1966).
Shidlovskaya, A. N., Syrkin, Ya. K., Novikov, S. S., Fainzil'berg, A. A., Sevost'yanova, V. V. & Gulevskaya, V. I. Dipole moments of some halogenated polynitroalkanes. Dokl. Akad. Nauk SSSR 132, 1367–1377 (1960).
Levow, T. E., Union Carbide Corporation. Novel organofunctional silicon compounds substituted with halogen and process for making same. US Patent 3694480 (1972).
Dakhis, M. I., Levin, A. A. & Shlyapochnikov, V. A. Equilibrium angles, barriers to internal rotation, and electronic structure of trinitromethane and its halo derivatives. Zh. Strukt. Khim. 14, 162–163 (1971).
Larkins, J. T., Nicholson, J. M. & Saalfeld, F. E. Negative and positive mass spectra of some substituted trinitromethanes. Org. Mass Spectrom. 5, 265–277 (1971).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 68, 441–451 (1964).
Zimmer, M. F., Barcody, E. E., Schwartz, M. & McAllister, M. P. Heat of formation of trinitrochloromethane by combustion calorimetry. J. Chem. Eng. Data 9, 527–529 (1964).
Reed, A. & Schleyer, P. v. R. The anomeric effect with central atoms other than carbon. 1. Strong interactions between nonbonded substituents in polyfluorinated first- and second-row hydrides. J. Am. Chem. Soc. 109, 7362–7373 (1987).
Reed, A. E., Curtiss, L. A. & Weinhold, F. Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem. Rev. 88, 899–926 (1988).
Harcourt, R. D. & Klapötke, T. M. Pauling three-electron bonds and increased valence structures as components of the “intellectual heritage” of qualitative valence bond theory. Res. Trends 9, 11–22 (2006).
Witt, J. R., Britton, D. & Mahon C. The crystal structures of BrC(CN)3, ClC(CN)3, and CH3C(CN)3 . Acta Crystallogr. B 28, 950–955 (1972).
Huntley, D. R., Markopoulos, G., Donovan, P. M., Scott, L. T. & Hoffmann, R. Squeezing C–C bonds. Angew. Chem. Int. Ed. 44, 7549–7553 (2005).
Schomaker, V. & Stevenson, D. R. Some revisions of the covalent radii and the additivity rule for the lengths of partially ionic single covalent bonds. J. Am. Chem. Soc. 63, 37–40 (1941).
Pritchard, H. O. & Skinner, H. A. The concept of electronegativity. Chem. Rev. 55, 745–786 (1955).
Hammet, L. P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 17, 125–136 (1935).
Hammet, L. P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 59, 96–103 (1937).
Taft, R. W. in Steric Effects in Organic Chemistry (ed. Newman, M. S.) Ch. 13 (Wiley, 1956).
Hine, J. & Bailey, W. C. Jr, Substituent constants for the trinitromethyl-, 1,1-dinitroethyl-, and related groups. J. Org. Chem. 26, 2098–2099 (1960).
Datta, D. Taft's substitutent constants, σ* and σI, and Huheey's group electronegativity. J. Phys. Org. Chem. 4, 96–100 (1991).
Huheey, J. E. Group electronegativity and polar substituent constants. J. Org. Chem. 31, 2365–2368 (1966).
Stewart, R. F. Valence structure from X-ray diffraction data: physical properties. J. Chem. Phys. 57, 1664–1668 (1972).
Politzer, P. & Truhlar, D. G. Chemical Applications of Atomic and Molecular Electrostatic Potentials (Plenum Press, 1981).
Bader, R. F. W., Carroll, M. T, Cheeseman, J. R. & Chang, C. Properties of atoms in molecules: atomic volumes. J. Am. Chem. Soc. 109, 7968–7979 (1987).
Politzer, P., Lane, P., Concha, M. C., Ma, Y. & Murray, J. S. An overview of halogen bonding. J. Mol. Model. 13, 305–311 (2007).
Murray, J. S., Lane, P. & Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 15, 723–729 (2009).
Clark, T., Hennemann, M., Murray, J. S. & Politzer, P. Halogen bonding: the σ-hole. J. Mol. Model. 13, 291–296 (2007).
Politzer, P., Murray, J. S., & Lane, P. σ-Hole bonding and hydrogen bonding: competitive interactions. Int. J. Quant. Chem. 107, 3046–3052 (2007).
Di Paolo, T. & Sandorfy, C. On the hydrogen bond breaking ability of fluorocarbons containing higher halogens. Can. J. Chem. 52, 3612–3622 (1974).
Rosenfield, R. E. Jr, Parthasarathy, R. & Dunitz, J. D. Directional preferences of nonbonded atomic contacts with divalent sulphur. 1. Electrophiles and nucleophiles. J. Am. Chem. Soc. 99, 4860–4862 (1977).
Murray-Rust, P. & Motherwell, W. D. S. Computer retrieval and analysis of molecular geometry 4. Intermolecular interactions. J. Am. Chem. Soc. 101, 4374–4376 (1979).
Auffinger, P., Hays, F. A., Westhof, E. & Shing Ho, P. Halogen bonds in biological molecules. Proc. Natl Acad. Sci. USA 101, 16789–16794 (2004).
Brinck T., Murray J. S. & Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quant. Chem., Quant. Biol. Symp. 19, 57–64 (1992).
Murray, J. S, Concha, M. C, Lane, P., Hobza, P. & Politzer, P. Blue shifts vs red shifts in σ-hole bonding. J. Mol. Model. 14, 699–704 (2008).
Wang, W., Wang, N. B., Zheng, W. & Tian, A. Theoretical study on the blue shifting halogen bond. J. Phys. Chem. A 108, 1799–1805 (2004).
Brändström, A. Prediction of Taft's σ* parameter for alkyl groups and alkyl groups containing polar substituents. J. Chem. Soc., Perkin Trans. 2, 1855–1857 (1999).
Hübschle, C. B. & Luger, P. MolIso – a program for colour-mapped iso-surfaces. J. Appl. Cryst. 39, 901–904 (2006).
Farrugia, L. J. ORTEP-3 for Windows—a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 30, 565 (1997).
Acknowledgements
Financial support of this work by the Ludwig-Maximilian University of Munich (LMU) and the Fonds der Chemischen Industrie is gratefully acknowledged. M.G. thanks the Cusanuswerk for a fellowship. P.P. and J.S.M. appreciate a Defence Threat Reduction Agency grant. We thank R. Harcourt for many inspired valence bond discussions.
Author information
Authors and Affiliations
Contributions
M.G. conceived, designed and carried out the experiment, analysed the data, interpreted and discussed the results and wrote the paper. B.H.T. provided the visualization of the molecular electrostatic potentials as well as NBO calculations. T.M.K. provided NBO calculations as well as the increased valence bond description and discussed the results. J.S.M. and P.P. provided the molecular electrostatic potential calculations, analysed the data, discussed the results and co-wrote the paper.
Corresponding authors
Supplementary information
Supplementary information
Supplementary information (PDF 398 kb)
Supplementary information
Crystallographic data for compound 1, chlorotrinitromethane (CIF 10 kb)
Rights and permissions
About this article
Cite this article
Göbel, M., Tchitchanov, B., Murray, J. et al. Chlorotrinitromethane and its exceptionally short carbon–chlorine bond. Nature Chem 1, 229–235 (2009). https://doi.org/10.1038/nchem.179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.179
This article is cited by
-
A promising high-energy-density material
Nature Communications (2017)
-
Modulating weak intramolecular interactions through the formation of beryllium bonds: complexes between squaric acid and BeH2
Journal of Molecular Modeling (2013)
-
Theoretical studies on the thermodynamic properties, densities, detonation properties, and pyrolysis mechanisms of trinitromethyl-substituted aminotetrazole compounds
Journal of Molecular Modeling (2013)
-
Trinitromethyl/trinitroethyl substituted CL-20 derivatives: structurally interesting and remarkably high energy
Journal of Molecular Modeling (2013)
-
Quantum chemical investigations on the structure–property relationship of aminopolynitrotriazoles
Structural Chemistry (2011)