Abstract
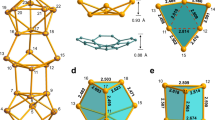
Although fullerenes were discovered nearly three decades ago, the mechanism of their formation remains a mystery. Many versions of the classic ‘bottom-up’ formation mechanism have been advanced, starting with C2 units that build up to form chains and rings of carbon atoms and ultimately form those well-known isolated fullerenes (for example, Ih-C60). In recent years, evidence from laboratory and interstellar observations has emerged to suggest a ‘top-down’ mechanism, whereby small isolated fullerenes are formed via shrinkage of giant fullerenes generated from graphene sheets. Here, we present molecular structural evidence for this top-down mechanism based on metal carbide metallofullerenes M2C2@C1(51383)-C84 (M = Y, Gd). We propose that the unique asymmetric C1(51383)-C84 cage with destabilizing fused pentagons is a preserved ‘missing link’ in the top-down mechanism, and in well-established rearrangement steps can form many well-known, high-symmetry fullerene structures that account for the majority of solvent-extractable metallofullerenes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kroto, H. W., Heath, J. R., O'Brien, S. C., Curl, R. F. & Smalley, R. E. C60-buckminsterfullerene. Nature 318, 162–163 (1985).
Ross, R. B. et al. Endohedral fullerenes for organic photovoltaic devices. Nature Mater. 8, 208–212 (2009).
Shultz, M. D. et al. Metallofullerene-based nanoplatform for brain tumor brachytherapy and longitudinal imaging in a murine orthotopic xenograft model. Radiology 261, 136–143 (2011).
Curl, R. F. On the formation of the fullerenes. Phil. Trans. R. Soc. Lond. A 343, 19–32 (1993).
Dunk, P. W. et al. Closed network growth of fullerenes. Nature Commun. 3, 855 (2012).
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nature Mater. 6, 183–191 (2007).
Chuvilin, A., Kaiser, U., Bichoutskaia, E., Besley, N. A. & Khlobystov, A. N. Direct transformation of graphene to fullerene. Nature Chem. 2, 450–453 (2010).
Cross, R. J. & Saunders, M. Transmutation of fullerenes. J. Am. Chem. Soc. 127, 3044–3047 (2005).
Cami, J., Bernard-Salas, J., Peeters, E. & Malek, S. E. Detection of C60 and C70 in a young planetary nebula. Science 329, 1180–1182 (2010).
Berné, O. & Tielens, A. G. G. M. F. Formation of buckminsterfullerene (C60) in interstellar space. Proc. Natl Acad. Sci. USA 109, 401–406 (2012).
Li, Z., Cheng, Z., Wang, R., Li, Q. & Fang, Y. Spontaneous formation of nanostructures in graphene. Nano Lett. 9, 3599–3602 (2009).
Shenoy, V. B., Reddy, C. D. & Zhang, Y.-W. Spontaneous curling of graphene sheets with reconstructed edges. ACS Nano 4, 4840–4844 (2010).
Irle, S., Zheng, G., Wang, Z. & Morokuma, K. The C60 formation puzzle ‘solved’: QM/MD simulations reveal the shrinking hot giant road of the dynamic fullerene self-assembly mechanism. J. Phys. Chem. B 110, 14531–14545 (2006).
Curl, R. F., Lee, M. K. & Scuseria, G. E. C60 buckminsterfullerene high yields unraveled. J. Phys. Chem. A 112, 11951–11955 (2008).
Fowler, P. W. & Manolopolous, D. E. in An Atlas of Fullerenes 2007 edn. (Dover Publications, 2007).
Murry, R. L., Strout, D. L., Odom, G. K. & Scuseria, G. E. Role of sp3 carbon and 7-membered rings in fullerene annealing and fragmentation. Nature 366, 665–667 (1993).
Troshin, P. A. et al. Isolation of two seven-membered ring C58 fullerene derivatives: C58F17CF3 and C58F18 . Science 309, 278–281 (2005).
Ioffe, I. N. et al. Chlorination of C86 to C84Cl32 with nonclassical heptagon-containing fullerene cage formed by cage shrinkage. Angew. Chem. Int. Ed. 49, 4784–4787 (2010).
Kroto, H. W. The stability of the fullerenes C24, C28, C32, C36, C50, C60 and C70 . Nature 329, 529–531 (1987).
Wang, C. R. et al. C66 fullerene encaging a scandium dimer. Nature 408, 426–427 (2000).
Stevenson, S. et al. A stable non-classical metallofullerene family. Nature 408, 427–428 (2000).
Tan, Y.-Z., Xie, S.-Y., Huang, R.-B. & Zheng, L.-S. The stabilization of fused-pentagon fullerene molecules. Nature Chem. 1, 450–460 (2009).
Shi, Z. Q., Wu, X., Wang, C. R., Lu, X. & Shinohara, H. Isolation and characterization of Sc2C2@C68: a metal-carbide endofullerene with a non-IPR carbon cage. Angew. Chem. Int. Ed. 45, 2107–2111 (2006).
Zheng, H., Zhao, X., Wang, W.-W., Yang, T. & Nagase, S. Sc2@C70 rather than Sc2C2@C68: density functional theory characterization of metallofullerene Sc2C70 . J. Chem. Phys. 137, 014308 (2012).
Zhang, J. et al. Nanoscale fullerene compression of an yttrium carbide cluster. J. Am. Chem. Soc. 134, 8487–8493 (2012).
Fu, W. et al. Gd2@C79N: isolation, characterization, and monoadduct formation of a very stable heterofullerene with a magnetic spin state of S = 15/2. J. Am. Chem. Soc. 33, 9741–9750 (2011).
Zhang, J. et al. Enhanced dipole moments in trimetallic nitride template endohedral metallofullerenes with the pentalene motif. J. Am. Chem. Soc. 135, 3351–3354 (2013).
Yang, T., Zhao, X., Li, S.-T. & Nagase, S. Is the isolated pentagon rule always satisfied for metallic carbide endohedral fullerenes? Inorg. Chem. 51, 11223–11225 (2012).
Fu, W. et al. 89Y and 13C NMR cluster and carbon cage studies of an yttrium metallofullerene family, Y3N@C2n (n = 40–43). J. Am. Chem. Soc. 131, 11762–11769 (2009).
Yang, H. et al. Detection of a family of gadolinium-containing endohedral fullerenes and the isolation and crystallographic characterization of one member as a metal carbide encapsulated inside a large fullerene cage. J. Am. Chem. Soc. 130, 17296–17300 (2008).
Rodriguez-Fortea, A., Alegret, N., Balch, A. L. & Poblet, J. M. The maximum pentagon separation rule provides a guideline for the structures of endohedral metallofullerenes. Nature Chem. 2, 955–961 (2010).
Mercado, B. Q. et al. Is the isolated pentagon rule merely a suggestion for endohedral fullerenes? The structure of a second egg-shaped endohedral fullerene—Gd3N@C s(39663)-C82 . 130, 7854–7855 (2008).
Beavers, C. M. et al. Tb3N@C84: an improbable, egg-shaped endohedral fullerene that violates the isolated pentagon rule. J. Am. Chem. Soc. 128, 11352–11353 (2006).
Tan, Y.-Z. et al. Carbon arc production of heptagon-containing fullerene C68 . Nature Commun. 2, 420 (2011).
Popov, A. A., Yang, S. & Dunsch, L. Endohedral fullerenes. Chem. Rev. 113, 5989–6113 (2013).
Inoue, T. et al. Trapping a C2 radical in endohedral metallofullerenes: synthesis and structures of (Y2C2)@C82 (isomers I, II, and III). J. Phys. Chem. B 108, 7573–7579 (2004).
Saha, B., Irle, S. & Morokuma, K. Hot giant fullerenes eject and capture C2 molecules: QM/MD simulations with constant density. J. Phys. Chem. C 115, 22707–22716 (2011).
Xie, S. Y. et al. Capturing the labile fullerene 50 as C50Cl10 . Science 304, 699 (2004).
Tan, Y.-Z. et al. An entrant of smaller fullerene: C56 captured by chlorines and aligned in linear chains. J. Am. Chem. Soc. 130, 15240–15241 (2008).
Ziegler, K., Mueller, A., Amsharov, K. Y. & Jansen, M. Capturing the most-stable C56 fullerene cage by in situ chlorination. Chem. Asian J. 6, 2412–2418 (2011).
Acknowledgements
The authors acknowledge support from the National Science Foundation (grants CHE-0938043 to H.C.D. and CHE-1011760 to A.L.B. and M.M.O.) and the Hollings Marine Laboratory NMR Facility. The authors also thank the Advanced Light Source, supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy (contract no. DE-AC02-05CH11231) for beam time, and S.J. Teat and C.M. Beavers for assistance.
Author information
Authors and Affiliations
Contributions
J.Z., Y.Y. and C.D. prepared the samples. F.L.B. and M.M.O. performed the crystal study. D.W.B. performed NMR characterization. T.F. performed DFT computations. W.K.R., R.F.H. and K.H. provided mass-spectral analysis. J.Z., M.M.O., A.L.B. and H.C.D analysed the results. J.Z. and H.C.D composed the manuscript and modified it based on critical comments from M.M.O. and A.L.B. The entire project was supervised by A.L.B. and H.C.D.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1902 kb)
Supplementary information
Crystallographic data for Gd2C2@C1(51383)-C84•Ni(OEP)•1.75(toluene)•0.25(benzene). (PDF 1902 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Bowles, F., Bearden, D. et al. A missing link in the transformation from asymmetric to symmetric metallofullerene cages implies a top-down fullerene formation mechanism. Nature Chem 5, 880–885 (2013). https://doi.org/10.1038/nchem.1748
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1748