Abstract
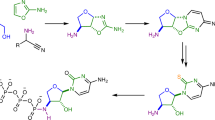
The recent synthesis of pyrimidine ribonucleoside-2′,3′-cyclic phosphates under prebiotically plausible conditions has strengthened the case for the involvement of ribonucleic acid (RNA) at an early stage in the origin of life. However, a prebiotic conversion of these weakly activated monomers, and their purine counterparts, to the 3′,5′-linked RNA polymers of extant biochemistry has been lacking (previous attempts led only to short oligomers with mixed linkages). Here we show that the 2′-hydroxyl group of oligoribonucleotide-3′-phosphates can be chemoselectively acetylated in water under prebiotically credible conditions, which allows rapid and efficient template-directed ligation. The 2′-O-acetyl group at the ligation junction of the product RNA strand can be removed under conditions that leave the internucleotide bonds intact. Remarkably, acetylation of mixed oligomers that possess either 2′- or 3′-terminal phosphates is selective for the 2′-hydroxyl group of the latter. This newly discovered chemistry thus suggests a prebiotic route from ribonucleoside-2′,3′-cyclic phosphates to predominantly 3′,5′-linked RNA via partially 2′-O-acetylated RNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Joyce, G. F. The antiquity of RNA-based evolution. Nature 418, 214–221 (2002).
Woese, C. The Genetic Code 179–195 (Harper & Row, 1967).
Crick, F. H. C. The origin of the genetic code. J. Mol. Biol. 38, 367–379 (1968).
Orgel, L. E. Evolution of the genetic apparatus. J. Mol. Biol. 38, 381–393 (1968).
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
Szostak, J. W. Systems chemistry on early Earth. Nature 459, 171–172 (2009).
Renz, M., Lohrmann, R. & Orgel, L. E. Catalysts for the polymerisation of adenosine cyclic 2′,3′-phosphate on a poly (U) template. Biochim. Biophys. Acta 240, 463–471 (1971).
Eftink, M. R. & Biltonen, R. L. Energetics of ribonuclease A catalysis. 2. Nonenzymatic hydrolysis of cytidine cyclic 2′,3′-phosphate. Biochemistry 22, 5134–5140 (1983).
Verlander, M. S., Lohrmann, R. & Orgel, L. E. Catalysts for the self-polymerization of adenosine cyclic 2′,3′-phosphate. J. Mol. Evol. 2, 303–316 (1973).
Verlander, M. S. & Orgel, L. E. Analysis of high molecular weight material from the polymerization of adenosine cyclic 2′,3′-phosphate. J. Mol. Evol. 3, 115–120 (1974).
Usher, D. A. & McHale, A. H. Nonenzymic joining of oligoadenylates on a polyuridylic acid template. Science 192, 53–54 (1976).
Bolli, M., Micura, R., Pitsch, S. & Eschenmoser, A. Pyranosyl-RNA: further observations on replication. Helv. Chim. Acta 80, 1901–1951 (1997).
Trevino, S. G., Zhang, N., Elenko, M. P., Lupták, A. & Szostak, J. W. Evolution of functional nucleic acids in the presence of nonheritable backbone heterogeneity. Proc. Natl Acad. Sci. USA 108, 13492–13497 (2011).
Kierzek, R., He, L. & Turner, D. H. Association of 2′-5′ oligoribonucleotides. Nucleic Acids Res. 20, 1685–1690 (1992).
Usher, D. A. Early chemical evolution of nucleic acids: a theoretical model. Science 196, 311–313 (1977).
Usher, D. A. & McHale, A. H. Hydrolytic stability of helical RNA: a selective advantage for the natural 3′,5′-bond. Proc. Natl Acad. Sci. USA 73, 1149–1153 (1976).
Rohatgi, R., Bartel, D. P. & Szostak, J. W. Nonenzymatic, template-directed ligation of oligoribonucleotides is highly regioselective for the formation of 3′−5′ phosphodiester bonds. J. Am. Chem. Soc. 118, 3340–3344 (1996).
Rammler, D. H., Lapidot, Y. & Khorana, H. G. Studies on polynucleotides. XIX. The specific synthesis of C3′-C5′ inter-ribonucleotidic linkage. A new approach and its use in the synthesis of C3′-C5′-linked uridine oligonucleotides. J. Am. Chem. Soc. 85, 1989–1997 (1963).
Coutsogeorgopoulos, C. & Khorana, H. G. Studies on polynucleotides. XXXI. The specific synthesis of C3′-C5′-linked ribopolynucleotides (6). A further study of the synthesis of uridine polynucleotides. J. Am. Chem. Soc. 86, 2926–2932 (1964).
Huber, C. & Wächtershäuser, G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 276, 245–247 (1997).
Loison, A., Dubant, S., Adam, P. & Albrecht, P. Elucidation of an iterative process of carbon–carbon bond formation of prebiotic significance. Astrobiology 10, 973–988 (2010).
de Duve, C. Blueprint for a Cell: the Nature and Origin of Life (Neil Patterson Publishers, 1991).
Hagan, W. J. Jr Uracil-catalyzed synthesis of acetyl phosphate: a photochemical driver for protometabolism. ChemBioChem 11, 383–387 (2010).
Liu, R. & Orgel, L. E. Oxidative acylation using thioacids. Nature 389, 52–54 (1997).
Biron, J-P., Parkes, A. L., Pascal, R. & Sutherland, J. D. Expeditious, potentially primordial, aminoacylation of nucleotides. Angew. Chem. Int. Ed. 44, 6731–6734 (2005).
Merino, E. J., Wilkinson, K. A., Coughlan, J. L. & Weeks, K. M. RNA structure analysis at single nucleotide resolution by selective 2′-hydroxyl acylation and primer extension (SHAPE). J. Am. Chem. Soc. 127, 4223–4231 (2005).
McGinnis, J. L., Dunkle, J. A., Cate, J. H. D. & Weeks, K. M. The mechanisms of RNA SHAPE chemistry. J. Am. Chem. Soc. 134, 6617–6624 (2012).
Gupta, S. C., Islam, N. B., Whalen, D. L., Yagi, H. & Jerina, D. M. Bifunctional catalysis in the nucleotide-catalyzed hydrolysis of (±)-7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. J. Org. Chem. 52, 3812–3815 (1987).
Cavalieri, L. F. Studies on the structure of nucleic acids. VII. On the identification of the isomeric cytidylic and adenylic acids. J. Am. Chem. Soc. 75, 5268–5270 (1953).
Rohatgi, R., Bartel, D. P. & Szostak, J. W. Kinetic and mechanistic analysis of nonenzymatic, template-directed oligoribonucleotide ligation. J. Am. Chem. Soc. 118, 3332–3339 (1996).
Goldsborough, A. S. Modified polynucleotides and uses thereof. US patent 6,867,290 (2005).
Nissen, P., Ippolito, J. A., Ban, N., Moore, P. B. & Steitz, T. A. RNA tertiary interactions in the large ribosomal subunit: the A-minor motif. Proc. Natl Acad. Sci. USA 98, 4899–4903 (2001).
Altona, C. & Sundaralingam, M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J. Am. Chem. Soc. 94, 8205–8212 (1972).
Guschlbauer, W. & Jankowski, K. Nucleoside conformation is determined by the electronegativity of the sugar substituent. Nucleic Acids Res. 8, 1421–1433 (1980).
Ferris, J. P., Huang, C-H. & Hagan, W. J. Jr N-cyanoimidazole and diimidazole imine: water-soluble condensing agents for the formation of the phosphodiester bond. Nucleos. Nucleot. 8, 407–414 (1989).
Kanaya, E. & Yanagawa, H. Template-directed polymerization of oligoadenylates using cyanogen bromide. Biochemistry 25, 7423–7430 (1986).
Horowitz, E. D. et al. Intercalation as a means to suppress cyclization and promote polymerization of base-pairing oligonucleotides in a prebiotic world. Proc. Natl Acad. Sci. USA 107, 5288–5293 (2010).
Acknowledgements
This work was funded by the Engineering and Physical Sciences Research Council through the provision of postdoctoral fellowships (C.D.D. and B.G.) and PhD studentships (M.W.P., S.I. and C.K.W.C.), the Medical Research Council through the provision of career development fellowships (F.R.B. and J.X., project no. MC_UP_A024_1009) and the Origin of Life Challenge, for which we thank H. Lonsdale. We thank C. Hilcenko, M. J. Churcher and V. B. Pinheiro for advice on polyacrylamide gel electrophoresis and fluorescence scanning, and D. Williams for advice on solid-phase oligonucleotide synthesis.
Author information
Authors and Affiliations
Contributions
F.R.B., C.K.W.C., C.D.D., B.G., S.I., M.W.P., J.D.S. and J.X. conceived and designed the experiments and analysed the data. F.R.B., C.K.W.C., C.D.D., B.G., S.I., M.W.P. and J.X. performed the experiments. F.R.B., S.I., M.W.P. and J.D.S. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5174 kb)
Rights and permissions
About this article
Cite this article
Bowler, F., Chan, C., Duffy, C. et al. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nature Chem 5, 383–389 (2013). https://doi.org/10.1038/nchem.1626
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1626
This article is cited by
-
Harnessing chemical energy for the activation and joining of prebiotic building blocks
Nature Chemistry (2020)
-
The chemistry and applications of RNA 2′-OH acylation
Nature Reviews Chemistry (2019)
-
Peptide ligation by chemoselective aminonitrile coupling in water
Nature (2019)
-
Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions
Nature Chemistry (2018)
-
Selective prebiotic conversion of pyrimidine and purine anhydronucleosides into Watson-Crick base-pairing arabino-furanosyl nucleosides in water
Nature Communications (2018)