Abstract
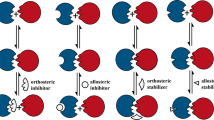
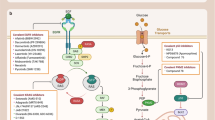
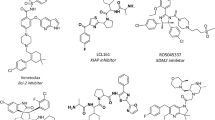
Inhibition of protein–protein interactions (PPIs) represents a significant challenge because it is unclear how they can be effectively and selectively targeted using small molecules. Achieving this goal is critical given the defining role of these interactions in biological processes. A rational approach to inhibitor design based on the secondary structure at the interface is the focus of much research, and different classes of designed ligands have emerged, some of which effectively and selectively disrupt targeted PPIs. This Review discusses the relevance of PPIs and, in particular, the importance of α-helix-mediated PPIs to chemical biology and drug discovery with a focus on designing inhibitors, including constrained peptides, foldamers and proteomimetic-derived ligands. In doing so, key challenges and major advances in developing generic approaches for the elaboration of PPI inhibitors are highlighted. The challenges faced in developing such ligands as drug leads — and how criteria applied to these may differ from conventional small-molecule drugs — are summarized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Stumpf, M. P. H. et al. Estimating the size of the human interactome. Proc. Natl Acad. Sci. USA 105, 6959–6964 (2008).
Berg, T. Modulation of protein-protein interactions with small organic molecules. Angew. Chem. Int. Ed. 42, 2462–2481 (2003).
Wells, J. A. & McLendon, C. L. Reaching for high-hanging fruit in drug discovery. Nature 450, 1001–1009 (2007).
Surade, S. & Blundell, T. L. Structural biology and drug discovery of difficult targets: the limits of ligandability. Chem. Biol. 19, 42–50 (2012).
Yin, H. & Hamilton, A. D. Strategies for targeting protein-protein interactions with synthetic agents. Angew. Chem. Int. Ed. 44, 4130–4163 (2005).
Wilson, A. J. Inhibition of protein-protein interactions using designed molecules. Chem. Soc. Rev. 38, 3289–3300 (2009).
Bullock, B. N., Jochim, A. L. & Arora, P. S. Assessing helical protein interfaces for inhibitor design. J. Am. Chem. Soc. 133, 14220–14223 (2011).
Edwards, T. & Wilson, A. Helix-mediated protein–protein interactions as targets for intervention using foldamers. Amino Acids 41, 743–754 (2011).
Henchey, L. K., Jochim, A. L. & Arora, P. S. Contemporary strategies for the stabilization of peptides in the α-helical conformation. Curr. Opin. Chem. Biol. 12, 692–697 (2008).
Davis, J. M., Tsou, L. K. & Hamilton, A. D. Synthetic non-peptide mimetics of alpha-helices. Chem. Soc. Rev. 36, 326–334 (2007).
Cummings, C. G. & Hamilton, A. D. Disrupting protein-protein interactions with non-peptidic, small molecule α-helix mimetics. Curr. Opin. Chem. Biol. 14, 341–346 (2010).
Stites, W. E. Protein-protein interactions: interface structure, binding thermodynamics, and mutational analysis. Chem. Rev. 97, 1233–1250 (1997).
Keskin, O., Gursoy, A., Ma, B. & Nussinov, R. Principles of protein-protein interactions: what are the preferred ways for proteins to interact. Chem. Rev. 108, 1225–1244 (2008).
Babine, R. E. & Bender, S. L. Molecular recognition of protein-ligand complexes: applications to drug design. Chem. Rev. 97, 1359–1472 (1997).
Clackson, T. & Wells, J. A. A hot-spot of binding-energy in a hormone-receptor interface. Science 267, 383–386 (1995).
Eyrisch, S. & Helms, V. Transient pockets on protein surfaces involved in protein-protein interaction. J. Med. Chem. 50, 3457–3464 (2007).
Kussie, P. H. et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274, 948–953 (1996).
Liu, X., Dai, S., Zhu, Y., Marrack, P. & Kappler, J. W. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity 19, 341–352 (2003).
Czabotar, P. E. et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc. Natl Acad. Sci. USA 104, 6217–6222 (2007).
Chen, L. et al. Differential targetting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393–403 (2005).
Chan, D. C., Fass, D., Berger, J. M. & Kim, P. S. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89, 263–273 (1997).
Bruning, J. B. et al. Coupling of receptor conformation and ligand orientation determine graded activity. Nature Chem. Biol. 6, 837–843 (2010).
Los, M., Roodhart, J. M. L. & Voest, E. E. Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. The Oncologist 12, 443–450 (2007).
Hudis, C. A. Trastuzumab - mechansim of action and use in clinical practice. New Engl. J. Med. 357, 39–51 (2007).
Biologic drugs set to top 2012 sales. Nature Med. 18, 636 (2012).
Chin, J. W. & Schepartz, A. Design and evolution of a miniature Bcl-2 binding protein. Angew. Chem. Int. Ed. 40, 3806–3809 (2001).
Rutledge, S. E., Volkman, H. M. & Schepartz, A. Molecular recognition of protein surfaces: high affinity ligands for the CBPKIX domain. J. Am. Chem. Soc. 125, 14336–14347 (2003).
Gemperli, A. C., Rutledge, S. E., Maranda, A. & Schepartz, A. Paralog-selective ligands for Bcl-2 proteins. J. Am. Chem. Soc. 127, 1596–1597 (2005).
Volkman, H. M., Rutledge, S. E. & Schepartz, A. Binding mode and transcriptional activation potential of high affinity ligands for the CBP KIX domain. J. Am. Chem. Soc. 127, 4649–4658 (2005).
Vaz, B. et al. Computational design, synthesis, and evaluation of miniproteins as androgen receptor coactivator mimics. Chem. Commun. 5377–5379 (2009).
Phan, T., Nguyen, H. D., Goksel, H., Mocklinghoff, S. & Brunsveld, L. Phage display selection of miniprotein binders of the estrogen receptor. Chem. Commun. 46, 8207–8209 (2010).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Grasberger, B. L. et al. Discovery and cocrystal structure of benzodiazepinedione HDM2 antagonists that activate p53 in cells. J. Med. Chem. 48, 909–912 (2005).
Shangary, S. et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc. Natl Acad. Sci. USA 105, 3933–3938 (2008).
Rew, Y. et al. Structure-based design of novel inhibitors of the MDM2–p53 interaction. J. Med. Chem. 55, 4936–4954 (2012).
Hajduk, P. J. & Greer, J. A decade of fragment-based drug design: strategic advances and lessons learned. Nature Rev. Drug Discov. 6, 211–219 (2007).
Bruncko, M. et al. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J. Med. Chem. 50, 641–662 (2007).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Zhou, H. et al. Design of Bcl-2 and Bcl-xl inhibitors with subnanomolar binding affinities based upon a new scaffold. J. Med. Chem. 55, 4664–4682 (2012).
Zhou, H. et al. Structure-based design of potent Bcl-2/Bcl-xL inhibitors with strong in vivo antitumor activity. J. Med. Chem. 55, 6149–6161 (2012).
Marshall, S. A., Lazar, G. A., Chirino, A. J. & Desjarlais, J. R. Rational design and engineering of therapeutic proteins. Drug Discov. Today 8, 212–221 (2003).
Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: a summary and pharmacological classification. Nature Rev. Drug Discov. 7, 21–39 (2008).
Scholtz, J. M. & Baldwin, R. L. The mechanism of alpha-helix formation by peptides. Annu. Rev. Biophys. Biomol. Struct. 21, 95–118 (1992).
Andrews, M. J. I. & Tabor, A. B. Forming stable helical peptides using natural and artificial amino acids. Tetrahedron 55, 11711–11743 (1999).
Garner, J. & Harding, M. M. Design and synthesis of α-helical peptides and mimetics. Org. Biomol. Chem. 5, 3577–3585 (2007).
Seebach, D. et al. Biological and pharmacokinetic studies with β-peptides. Chimia 52, 734–739 (1998).
Glickson, J. D. & Applequi, J. Conformation of poly-beta-alanine in aqueous solution from proton magnetic resonance and deuterium exchange studies. J. Am. Chem. Soc. 93, 3276–3281 (1971).
Gellman, S. H. Foldamers: A manifesto. Acc. Chem. Res. 31, 173–180 (1998).
Cheng, R. P., Gellman, S. H. & DeGrado, W. F. β-Peptides: from structure to function. Chem. Rev. 101, 3219–3232 (2001).
Pellegrini, M., Royo, M., Chorev, M. & Mierke, D. F. Conformational consequences of i, i+3 cystine linkages: Nucleation for α-helicity? J. Pept. Res. 49, 404–414 (1997).
Galande, A. K. et al. Potent inhibitors of LXXLL-based protein-protein interactions. ChemBioChem 6, 1991–1998 (2005).
Leduc, A. M. et al. Helix-stabilized cyclic peptides as selective inhibitors of steroid receptor-coactivator interactions. Proc. Natl Acad. Sci. USA 100, 11273–11278 (2003).
Galande, A. K., Bramlett, K. S., Burris, T. P., Wittliff, J. L. & Spatola, A. F. Thioether side chain cyclization for helical peptide formation: Inhibitors of estrogen receptor-coactivator interactions. J. Pept. Res. 63, 297–302 (2004).
Chorev, M. et al. Cyclic parathyroid-hormone related protein antagonists - lysine-13 to aspartic-acid 17 i to (i + 4) side-chain to side-chain lactamization. Biochemistry 30, 5968–5974 (1991).
Geistlinger, T. R. & Guy, R. K. Novel selective inhibitors of the interaction of individual nuclear hormone receptors with a mutually shared steroid receptor coactivator 2. J. Am. Chem. Soc. 125, 6852–6853 (2003).
Judice, J. K. et al. Inhibition of HIV Type 1 infectivity by constrained alpha-helical peptides: implications for the viral fusion mechanism. Proc. Natl Acad. Sci. USA 94, 13426–13430 (1997).
Sia, S. K., Carr, P. A., Cochran, A. G., Malashkevich, V. N. & Kim, P. S. Short constrained peptides that Inhibit HIV-1 entry. Proc. Natl Acad. Sci. USA 99, 14664–14669 (2002).
Harrison, R. S. et al. Downsizing human, bacterial, and viral proteins to short water-stable alpha helices that maintain biological potency. Proc. Natl Acad. Sci. USA 107, 11686–11691 (2010).
Blackwell, H. E. & Grubbs, R. H. Highly efficient synthesis of covalently cross-linked peptide helices by ring-closing metathesis. Angew. Chem. Int. Ed. 37, 3281–3284 (1998).
Schafmeister, C. E., Po, J. & Verdine, G. L. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J. Am. Chem. Soc. 122, 5891–5892 (2000).
Walensky, L. D. et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Walensky, L. D. et al. A stapled BID BH3 helix directly binds and activates Bax. Mol. Cell 24, 199–210 (2006).
Braun, C. R. et al. Photoreactive stapled BH3 peptides to dissect the BCL-2 family interactome. Chem. Biol. 17, 1325–1333 (2010).
Stewart, M. L., Fire, E., Keating, A. E. & Walensky, L. D. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nature Chem. Biol. 6, 595–601 (2010).
Bernal, F. et al. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer Cell 18, 411–422 (2010).
Baek, S. et al. Structure of the stapled p53 peptide bound to Mdm2. J. Am. Chem. Soc. 134, 103–106 (2011).
Phillips, C. et al. Design and structure of stapled peptides binding to estrogen receptors. J. Am. Chem. Soc. 133, 9696–9699 (2011).
Moellering, R. E. et al. Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182–188 (2009).
Bird, G. H. et al. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Natl Acad. Sci. USA 107, 14093–14098 (2010).
Cabezas, E. & Satterthwait, A. C. The hydrogen-bond mimic approach: solid phase synthesis of a peptide stabilized as an α-helix with a hydrazone link. J. Am. Chem. Soc. 121, 3862–3875 (1999).
Patgiri, A., Jochim, A. L. & Arora, P. S. A Hydrogen-bond surrogate approach for stabilization of short peptide sequences in α-helical conformation. Acc. Chem. Res. 41, 1289–1300 (2008).
Chapman, R. N., Dimartino, G. & Arora, P. S. A highly stable short α-helix constrained by a main-chain hydrogen-bond surrogate. J. Am. Chem. Soc. 126, 12252–12253 (2004).
Wang, D. Y., Liao, W. & Arora, P. S. Binding properties of artificial α-helices derived from a hydrogen-bond surrogate: application to Bcl-xL. Angew. Chem. Int. Ed. 44, 6525–6529 (2005).
Wang, D., Lu, M. & Arora, P. S. Inhibition of HIV-1 fusion by hydrogen-bond-surrogate-based α-helices. Angew. Chem. Int. Ed. 47, 1879–1882 (2008).
Henchey, L. K., Porter, J. R., Ghosh, I. & Arora, P. S. High specificity in protein recognition by hydrogen-bond-surrogate α-helices: selective inhibition of the p53/MDM2 complex. ChemBioChem 11, 2104–2107 (2010).
Henchey, L. K. et al. Inhibition of hypoxia inducible factor 1-transcription coactivator interaction by a hydrogen bond surrogate α-helix. J. Am. Chem. Soc. 132, 941–943 (2010).
Patgiri, A., Yadav, K. K., Arora, P. S. & Bar-Sagi, D. An orthosteric inhibitor of the Ras-Sos interaction. Nature Chem. Biol. 7, 585–587 (2011).
Mahon, A. B. & Arora, P. S. Design, synthesis and protein-targeting properties of thioether-linked hydrogen bond surrogate helices. Chem. Commun. 1416–1418 (2011).
Muppidi, A. et al. Rational design of proteolytically stable, cell-permeable peptide-based selective Mcl-1 inhibitors. J. Am. Chem. Soc. 134, 14734–14737 (2012).
Jo, H. et al. Development of α-helical calpain probes by mimicking a natural protein-protein interaction. J. Am. Chem. Soc. 134, 17704–17713 (2012).
Kneissl, S., Loveridge, E. J., Williams, C., Crump, M. P. & Allemann, R. K. Photocontrollable peptide-based switches target the anti-apoptotic protein Bcl-xL . ChemBioChem 9, 3046–3054 (2008).
Wysoczanski, P. et al. NMR solution structure of a photoswitchable apoptosis activating bak peptide bound to Bcl-xL . J. Am. Chem. Soc. 134, 7644–7647 (2012).
English, E. P., Chumanov, R. S., Gellman, S. H. & Compton, T. Rational development of β-peptide inhibitors of human cytomegalovirus entry. J. Biol. Chem. 281, 2661–2667 (2006).
Kritzer, J. A., Lear, J. D., Hodsdon, M. E. & Schepartz, A. Helical β-peptide inhibitors of the p53-hDM2 interaction. J. Am. Chem. Soc. 126, 9468–9469 (2004).
Harker, E. A., Daniels, D. S., Guarracino, D. A. & Schepartz, A. β-Peptides with improved affinity for hDM2 and hDMX. Bioorg. Med. Chem. 17, 2038–2046 (2009).
Hintersteiner, M. et al. A highly potent and cellularly active β-peptidic inhibitor of the p53/hDM2 interaction. ChemBioChem 10, 994–998 (2009).
Stephens, O. M. et al. Inhibiting HIV fusion with a β-peptide foldamer. J. Am. Chem. Soc. 127, 13126–13127 (2005).
Acton, S. et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271, 518–520 (1996).
Werder, M., Hauser, H., Abele, S. & Seebach, D. β-Peptides as inhibitors of small-intestinal cholesterol and fat absorption. Helv. Chim. Acta 82, 1774–1783 (1999).
Sadowsky, J. D. et al. (α/β+α)-Peptide antagonists of BH3 domain/Bcl-xL recognition: Toward general strategies for foldamer-based inhibition of protein-protein interactions. J. Am. Chem. Soc. 129, 139–154 (2007).
Lee, E. F. et al. High-resolution structural characterization of a helical α/β-peptide foldamer bound to the anti-apoptotic protein Bcl-xL . Angew. Chem. Int. Ed. 48, 4318–4322 (2009).
Horne, W. S., Boersma, M. D., Windsor, M. A. & Gellman, S. H. Sequence-based design of α/β-peptide foldamers that mimic BH3 domains. Angew. Chem. Int. Ed. 47, 2853–2856 (2008).
Lee, E. F. et al. Structural basis of Bcl-xL recognition by a BH3-mimetic α/β-peptide generated by sequence-based design. ChemBioChem 12, 2025–2032 (2011).
Boersma, M. D. et al. Evaluation of diverse α/β-backbone patterns for functional α-helix mimicry: Analogues of the Bim BH3 domain. J. Am. Chem. Soc. 134, 315–323 (2011).
Horne, W. S. et al. Structural and biological mimicry of protein surface recognition by α/β-peptide foldamers. Proc. Natl Acad. Sci. USA 106, 14751–14756 (2009).
Johnson, L. M. et al. Enhancement of α-helix mimicry by an α/β-peptide foldamer via incorporation of a dense ionic side-chain array. J. Am. Chem. Soc. 134, 7317–7320 (2012).
Hara, T., Durell, S. R., Myers, M. C. & Appella, D. H. Probing the structural requirements of peptoids that inhibit hDM2−p53 interactions. J. Am. Chem. Soc. 128, 1995–2004 (2006).
Hayashi, R. et al. N-Acylpolyamine inhibitors of HDM2 and HDMX binding to p53. Bioorg. Med. Chem. 17, 7884–7893 (2009).
Sawada, T. & Gellman, S. H. Structural mimicry of the α-helix in aqueous solution with an isoatomic α/β/γ-peptide backbone. J. Am. Chem. Soc. 133, 7336–7339 (2011).
Patgiri, A., Joy, S. T. & Arora, P. S. Nucleation effects in peptide foldamers. J. Am. Chem. Soc. 134, 11495–11502 (2012).
Fasan, R. et al. Structure–activity studies in a family of β-hairpin protein epitope mimetic inhibitors of the p53–HDM2 protein–protein interaction. ChemBioChem 7, 515–526 (2006).
Ripka, A. S. & Rich, D. H. Peptidomimetic design. Curr. Opin. Chem. Biol. 2, 441–452 (1998).
Nolan, W. P., Ratcliffe, G. S. & Rees, D. C. The synthesis of 1,6-disubstituted indanes which mimic the orientation of amino acid side-chains in a protein alpha-helix motif. Tetrahedron Lett. 33, 6879–6882 (1992).
Horwell, D. C., Howson, W., Ratcliffe, G. S. & Willems, H. M. G. The design of dipeptide helical mimetics: The synthesis tachykinin receptor affinity and conformational analysis of 1,1,6-trisubstituted indanes. Bioorg. Med. Chem. 4, 33–42 (1996).
Orner, B. P., Ernst, J. T. & Hamilton, A. D. Toward proteomimetics: Terphenyl derivatives as structural and functional mimics of extended regions of an α-helix. J. Am. Chem. Soc. 123, 5382–5383 (2001).
Ernst, J. T. et al. Design of a protein surface antagonist based on α-helix mimicry: Inhibition of gp41 assembly and viral fusion. Angew. Chem. Int. Ed. 41, 278–282 (2002).
Kutzki, O. et al. Development of a potent Bcl-xL antagonist based on α-helix mimicry. J. Am. Chem. Soc. 124, 11838–11839 (2002).
Yin, H. et al. Terphenyl-based Bak BH3 α-helical proteomimetics as low-molecular-weight antagonists of Bcl-xL . J. Am. Chem. Soc. 127, 10191–10196 (2005).
Kazi, A. et al. The BH3 α-helical mimic BH3-M6 disrupts Bcl-xL, Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J. Biol. Chem. 286, 9382–9392 (2011).
Yin, H. et al. Terephthalamide derivatives as mimetics of helical peptides: Disruption of the Bcl-xL/Bak interaction. J. Am. Chem. Soc. 127, 5463–5468 (2005).
Rodriguez, J. M., Nevola, L., Ross, N. T., Lee, G.-i. & Hamilton, A. D. Synthetic inhibitors of extended helix–protein interactions based on a biphenyl 4,4′-dicarboxamide scaffold. ChemBioChem 10, 829–833 (2009).
Rodriguez, J. M. & Hamilton, A. D. Benzoylurea oligomers: synthetic foldamers that mimic extended α-helices. Angew. Chem. Int. Ed. 46, 8614–8617 (2007).
Rodriguez, J. M. et al. Structure and function of benzoylurea-derived α-helix mimetics targeting the Bcl-xL/Bak binding interface. ChemMedChem 4, 649–656 (2009).
Biros, S. M. et al. Heterocyclic α-helix mimetics for targeting protein-protein interactions. Bioorg. Med. Chem. Lett. 17, 4641–4645 (2007).
Cummings, C. G., Ross, N. T., Katt, W. P. & Hamilton, A. D. Synthesis and biological evaluation of a 5-6-5 imidazole-phenyl-thiazole based α-helix mimetic. Org. Lett. 11, 25–28 (2009).
Huc, I. Aromatic oligoamide foldamers. Eur. J. Org. Chem. 2004, 17–29 (2004).
Ernst, J. T., Becerril, J., Park, H. S., Yin, H. & Hamilton, A. D. Design and application of an α-helix-mimetic scaffold based on an oligoamide-foldamer strategy: antagonism of the Bak BH3/Bcl-xL complex. Angew. Chem. Int. Ed. 42, 535–539 (2003).
Saraogi, I. et al. Synthetic α-helix mimetics as agonists and antagonists of islet amyloid polypeptide aggregation. Angew. Chem. Int. Ed. 49, 736–739 (2010).
Yin, H., Frederick, K. K., Liu, D., Wand, A. J. & DeGrado, W. F. Arylamide derivatives as peptidomimetic inhibitors of calmodulin. Org. Lett. 8, 223–225 (2005).
Ahn, J. M. & Han, S. Y. Facile Synthesis of benzamides to mimic an α-helix. Tetrahedron Lett. 48, 3543–3547 (2007).
Plante, J. et al. Synthesis of functionalised aromatic oligamide rods. Org. Biomol. Chem. 6, 138–146 (2008).
Saraogi, I., Incarvito, C. D. & Hamilton, A. D. Controlling curvature in a family of oligoamide α-helix mimetics. Angew. Chem. Int. Ed. 47, 9691–9694 (2008).
Yap, J. L. et al. Relaxation of the rigid backbone of an oligoamide-foldamer-based α-helix mimetic: Identification of potent Bcl-xL inhibitors. Org. Biomol. Chem. 10, 2928–2933 (2012).
Plante, J. P. et al. Oligobenzamide proteomimetic inhibitors of the p53-hDM2 protein-protein interaction. Chem. Commun. 5091–5093 (2009).
Shaginian, A. et al. Design, synthesis, and evaluation of an α-helix mimetic library targeting protein–protein interactions. J. Am. Chem. Soc. 131, 5564–5572 (2009).
Whitby, L. R. et al. Discovery of HIV fusion inhibitors targeting gp41 using a comprehensive α-helix mimetic library. Bioorg. Med. Chem. Lett. 22, 2861–2865 (2012).
Azzarito, V. et al. 2-O-Alkylated para-benzamide α-helix mimetics: The role of scaffold curvature. Org. Biomol. Chem. 10, 6469–6472 (2012).
Lu, F. et al. Proteomimetic libraries: Design, synthesis, and evaluation of p53−MDM2 interaction inhibitors. J. Comb. Chem. 8, 315–325 (2006).
Shahian, T. et al. Inhibition of a viral enzyme by a small-molecule dimer disruptor. Nature Chem. Biol. 5, 640–646 (2009).
Campbell, F., Plante, J. P., Edwards, T. A., Warriner, S. L. & Wilson, A. J. N-Alkylated oligoamide α-helical proteomimetics. Org. Biomol. Chem. 8, 2344 (2010).
Long, K., Edwards, T. A. & Wilson, A. J. Microwave assisted solid phase synthesis of highly functionalized N-alkylated oligobenzamide α-helix mimetics. Biorg. Med. Chem. http://dx.doi.org/10.1016/j.bmc.2012.09.053 (2012).
Lee, J. H. et al. Novel pyrrolopyrimidine-based α-helix mimetics: Cell-permeable inhibitors of protein-protein interactions. J. Am. Chem. Soc. 133, 676–679 (2011).
Kim, I. C. & Hamilton, A. D. Diphenylindane-based proteomimetics reproduce the projection of the i, i+3, i+4, and i+7 residues on an α-helix. Org. Lett. 8, 1751–1754 (2006).
Marimganti, S., Cheemala, M. N. & Ahn, J. M. Novel amphiphilic alpha-helix mimetics based on a bis-benzamide scaffold. Org. Lett. 11, 4418–4421 (2009).
Tošovská, P. & Arora, P. S. Oligooxopiperazines as nonpeptidic α-helix mimetics. Org. Lett. 12, 1588–1591 (2010).
Becerril, J. & Hamilton, A. D. Helix mimetics as inhibitors of the interaction of the estrogen receptor with coactivator peptides. Angew. Chem. Int. Ed. 46, 4471–4473 (2007).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 23, 3–25 (1997).
Lichtiger, S. et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. New Engl. J. Med. 330, 1841–1845 (1994).
Acknowledgements
The authors would like to acknowledge the financial support of the European Research Council (ERC-StG-240324) V.A. and A.J.W. Yorkshire Cancer Research (L348) K.L. and A.J.W. N.S.M. would like to acknowledge the University of Leeds for a Mary and Alice Smith Endowed Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Azzarito, V., Long, K., Murphy, N. et al. Inhibition of α-helix-mediated protein–protein interactions using designed molecules. Nature Chem 5, 161–173 (2013). https://doi.org/10.1038/nchem.1568
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1568
This article is cited by
-
NLRP6 potentiates PI3K/AKT signalling by promoting autophagic degradation of p85α to drive tumorigenesis
Nature Communications (2023)
-
Synthesis of new hybrid indolyl-pyridines with sulfonamide moiety in the presence of Fe3O4@SiO2@(CH2)3-urea-quinolinium trifluoroacetate via a cooperative vinylogous anomeric-based oxidation
Journal of the Iranian Chemical Society (2023)
-
A novel mRNA decay inhibitor abolishes pathophysiological cellular transition
Cell Death Discovery (2022)
-
Binary combinatorial scanning reveals potent poly-alanine-substituted inhibitors of protein-protein interactions
Communications Chemistry (2022)
-
Design of peptide-based foldamers for inhibiting the Hdm2-p53 interaction using backbone modification: a molecular dynamics study
Journal of the Iranian Chemical Society (2022)