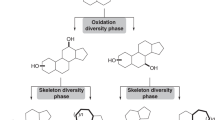
Abstract
High-throughput screening is the dominant method used to identify lead compounds in drug discovery. As such, the makeup of screening libraries largely dictates the biological targets that can be modulated and the therapeutics that can be developed. Unfortunately, most compound-screening collections consist principally of planar molecules with little structural or stereochemical complexity, compounds that do not offer the arrangement of chemical functionality necessary for the modulation of many drug targets. Here we describe a novel, general and facile strategy for the creation of diverse compounds with high structural and stereochemical complexity using readily available natural products as synthetic starting points. We show through the evaluation of chemical properties (which include fraction of sp3 carbons, ClogP and the number of stereogenic centres) that these compounds are significantly more complex and diverse than those in standard screening collections, and we give guidelines for the application of this strategy to any suitable natural product.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Swinney, D. C. & Anthony, J. How were new medicines discovered? Nature Rev. Drug Discov. 10, 507–519 (2011).
Flaherty, K. T., Yasothan, U. & Kirkpatrick, P. Vemurafenib. Nature Rev. Drug Discov. 10, 811–812 (2011).
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl Acad. Sci. USA 105, 3041–3046 (2008).
Domling, A. Small molecular weight protein–protein interaction antagonists: an insurmountable challenge? Curr. Opin. Chem. Biol. 12, 281–291 (2008).
Grivas, P. D., Kiaris, H. & Papavassiliou, A. G. Tackling transcription factors: challenges in antitumor therapy. Trends Mol. Med. 17, 537–538 (2011).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nature Rev. Drug Discov. 6, 29–40 (2007).
Kodadek, T. The rise, fall and reinvention of combinatorial chemistry. Chem. Commun. 47, 9757–9763 (2011).
Schreiber, S. L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287, 1964–1969 (2000).
Galloway, W. R., Isidro-Llobet, A. & Spring, D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nature Commun. 1, 80 (2010).
Spaller, M. R., Burger, M. T., Fardis, M. & Bartlett, P. A. Synthetic strategies in combinatorial chemistry. Curr. Opin. Chem. Biol. 1, 47–53 (1997).
Clemons, P. A. et al. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc. Natl Acad. Sci. USA 107, 18787–18792 (2010).
Cui, J. et al. Creation and manipulation of common functional groups en route to a skeletally diverse chemical library. Proc. Natl Acad. Sci. USA 108, 6763–6768 (2011).
Pelish, H. E., Westwood, N. J., Feng, Y., Kirchhausen, T. & Shair, M. D. Use of biomimetic diversity-oriented synthesis to discover galanthamine-like molecules with biological properties beyond those of the natural product. J. Am. Chem. Soc. 123, 6740–6741 (2001).
Goess, B. C., Hannoush, R. N., Chan, L. K., Kirchhausen, T. & Shair, M. D. Synthesis of a 10,000-membered library of molecules resembling carpanone and discovery of vesicular traffic inhibitors. J. Am. Chem. Soc. 128, 5391–5403 (2006).
Kumar, N., Kiuchi, M., Tallarico, J. A. & Schreiber, S. L. Small-molecule diversity using a skeletal transformation strategy. Org. Lett. 7, 2535–2538 (2005).
Balthaser, B. R., Maloney, M. C., Beeler, A. B., Porco, J. A. & Snyder, J. K. Remodelling of the natural product fumagillol employing a reaction discovery approach. Nature Chem. 3, 969–973 (2011).
Kopp, F., Stratton, C. F., Akella, L. B. & Tan, D. S. A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion. Nature Chem. Biol. 8, 358–365 (2012).
Niggemann, J., Michaelis, K., Frank, R., Zander, N. & Hofle, G. Natural product-derived building blocks for combinatorial synthesis. Part 1. Fragmentation of natural products from myxobacteria. J. Chem. Soc. Perkin Trans. 1, 2490–2503 (2002).
Lachance, H., Wetzel, S., Kumar, K. & Waldmann, H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 55, 5989–6001 (2012).
Aquino, C. et al. A biomimetic polyketide-inspired approach to small-molecule ligand discovery. Nature Chem. 4, 99–104 (2012).
O'Connor, S. E. & Maresh, J. J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 23, 532–547 (2006).
Curtis, P. J. & Cross, B. E. Gibberellic acid. A new metabolite from the culture of filtrates of Gibberella fujikuroi. Chem. Ind. 1066 (1954).
Rodrigues, C., Vandenberghe, L. P., de Oliveira, J. & Soccol, C. R. New perspectives of gibberellic acid production: a review. Crit. Rev. Biotechnol. 32, 263–273 (2012).
Cross, B. E. Gibberellic acid. Part I. J. Chem. Soc. 4670–4676 (1954).
Mulholland, T. P. C. Gibberellic acid. Part 9. The structure of allogibberic acid. J. Chem. Soc. 2693–2701 (1958).
Henderson, J. H. & Graham, H. D. A possible mechanism for biological and chemical activity of gibberellic acid. Nature 193, 1055–1056 (1962).
Grove, J. F. & Mulholland, T. P. C. Gibberellic acid. Part 12. The stereochemistry of allogibberic acid. J. Chem. Soc. 3007–3022 (1960).
Cross, B. E., Grove, J. F. & Morrison, A. Gibberellic acid. 18. Some rearrangements of ring A. J. Chem. Soc. 2498–2515 (1961).
Cross, B. E. & Markwell, R. E. Rearrangements of the gibbane skeleton during reactions with 2,3-dichloro-5,6-dicyanobenzoquinone. J. Chem. Soc. Perkin Trans. I 1476–1487 (1973).
Idler, D. R., Schmidt, P. J. & Bitners, I. Isolation and identification of adrenosterone in salmon (Oncorhynchus nerka) plasma. Can. J. Biochem. Physiol. 39, 1653–1654 (1961).
Borthakur, M. & Boruah, R. C. A microwave promoted and Lewis acid catalysed solventless approach to 4-azasteroids. Steroids 73, 637–641 (2008).
Bernstein, S., Littell, R. & Williams, J. H. Steroidal cyclic ketals. IV. The conversion of 11-keto- to 11α-hydroxysteroids. The preparation of 11-epi-hydrocortisone, and Δ4-androstene-11α-ol-3,17-dione. J. Am. Chem. Soc. 75, 1481–1482 (1953).
Lecomte, V., Stephan, E., LeBideau, F. & Jaouen, G. Improved addition of organolithium reagents to hindered and/or enolisable ketones. Tetrahedron 59, 2169–2176 (2003).
Stephan, E., Brossat, M., Lecomte, V. & Bouit, P. A. Synthesis of the 11 beta-hydroxymethyl-androst-4-en-3,17-dione. Tetrahedron 62, 3052–3055 (2006).
Song, C. E. Cinchona Alkaloids in Synthesis and Catalysis 1–10 (Wiley, 2009).
Smith, A. C. & Williams, R. M. Rabe rest in peace: confirmation of the rabe-kindler conversion of D-quinotoxine into quinine: experimental affirmation of the Woodward–Doering formal total synthesis of quinine. Angew. Chem. Int. Ed. 47, 1736–1740 (2008).
Hintermann, L., Schmitz, M. & Englert, U. Nucleophilic addition of organometallic reagents to cinchona alkaloids: simple access to diverse architectures. Angew. Chem. Int. Ed. 46, 5164–5167 (2007).
Baell, J. B. & Holloway, G. A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 53, 2719–2740 (2010).
O'Shea, R. & Moser, H. E. Physicochemical properties of antibacterial compounds: implications for drug discovery. J. Med. Chem. 51, 2871–2878 (2008).
Walters, W. P., Green, J., Weiss, J. R. & Murcko, M. A. What do medicinal chemists actually make? A 50-year retrospective. J. Med. Chem. 54, 6405–6416 (2011).
Feher, M. & Schmidt, J. M. Property distributions: differences between drugs, natural products, and molecules from combinatorial chemistry. J. Chem. Inf. Comput. Sci. 43, 218–227 (2003).
Yan, A. & Gasteiger, J. Prediction of aqueous solubility of organic compounds by topological descriptors. QSAR Comb. Sci. 22, 821–829 (2003).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Leeson, P. D. & St-Gallay, S. A. The influence of the ‘organizational factor’ on compound quality in drug discovery. Nature Rev. Drug Discov. 10, 749–765 (2011).
Leeson, P. D. & Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nature Rev. Drug Discov. 6, 881–890 (2007).
Rogers, D. J. & Tanimoto, T. T. A computer program for classifying plants. Science 132, 1115–1118 (1960).
Rogers, D. & Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 50, 742–754 (2010).
Huggins, D. J., Venkitaraman, A. R. & Spring, D. R. Rational methods for the selection of diverse screening compounds. ACS Chem. Biol. 6, 208–217 (2011).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Natural Prod. 75, 311–335 (2012).
Acknowledgements
We thank the Office of Naval Research (N00014-09-1-0249) and the University of Illinois for support of this work. R.W.H. III is an American Cancer Society postdoctoral fellow. K.C.M. is a National Science Foundation predoctoral fellow and a Robert C. and Carolyn J. Springborn graduate fellow. M.F.R. is a member of the National Institutes of Health Chemistry–Biology Interface (Training Grant NRSA 1-T-32-GM070421). We thank D. Gray for the X-ray analysis of Q1, M. Brichacek for technical assistance with selected two-dimensional NMR experiments in addition to many helpful discussions and R. J. Rafferty for technical assistance. We also thank M. Ahmed and A. Al-khouja for the synthesis of various intermediates during these investigations.
Author information
Authors and Affiliations
Contributions
P.J.H. and R.W.H. III conceived the study. R.W.H. III designed and executed the synthesis of the A compound set and constructed libraries based on compounds A1, A2, A3, A5, A9, A12 and A15. K.C.M. designed and executed the synthesis of the G compound set and constructed libraries based on G6, G10 and G19. R.W.H. designed and executed the synthesis of the Q compound set and constructed the library based on Q1. T.A.F. performed computational analyses of all the compound sets discussed in this Article. M.F.R. constructed libraries of compounds A3, A12, G10 and G16. P.J.H. supervised this research and wrote this manuscript with the assistance of R.W.H. III, K.C.M., R.W.H. and T.A.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 11000 kb)
Supplementary information
Crystallographic data for compound Q1 (CIF 31 kb)
Rights and permissions
About this article
Cite this article
Huigens III, R., Morrison, K., Hicklin, R. et al. A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nature Chem 5, 195–202 (2013). https://doi.org/10.1038/nchem.1549
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1549
This article is cited by
-
Porin-independent accumulation in Pseudomonas enables antibiotic discovery
Nature (2023)
-
An unexpected synthesis of azepinone derivatives through a metal-free photochemical cascade reaction
Nature Communications (2023)
-
Single-atom skeletal editing of 2H-indazoles enabled by difluorocarbene
Science China Chemistry (2023)
-
Combining visible-light induction and copper catalysis for chemo-selective nitrene transfer for late-stage amination of natural products
Communications Chemistry (2022)
-
Azoarene activation for Schmidt-type reaction and mechanistic insights
Nature Communications (2022)