Abstract
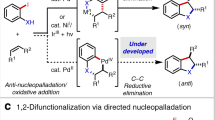
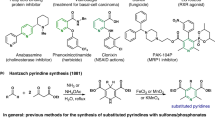
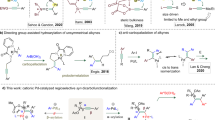
The pyridine heterocycle continues to play a vital role in the development of human medicines. More than 100 currently marketed drugs contain this privileged unit, which remains highly sought after synthetically. We report an efficient means to access di- and trisubstituted pyridines in an efficient and highly controlled manner using transient 3,4-pyridyne intermediates. Previous efforts to employ 3,4-pyridynes for the construction of substituted pyridines were hampered by a lack of regiocontrol or the inability to later manipulate an adjacent directing group. The strategy relies on the use of proximal halide or sulfamate substituents to perturb pyridyne distortion, which in turn governs regioselectivities in nucleophilic addition and cycloaddition reactions. After trapping of the pyridynes generated in situ, the neighbouring directing groups may be removed or exploited using versatile metal-catalysed cross-coupling reactions. This methodology now renders 3,4-pyridynes as useful synthetic building blocks for the creation of highly decorated derivatives of the medicinally privileged pyridine heterocycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pozharskii, A. F., Soldatenkov, A. & Katritzky, A. R. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry, and Applications 2nd edn (Wiley, 2011).
Joule, J. A. & Mills, K. Heterocyclic Chemistry 5th edn (Wiley, 2010).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Synthesis of dihydropyridines and pyridines from imines and alkynes via C–H activation. J. Am. Chem. Soc. 130, 3645–3651 (2008).
Fischer, D. F. & Sarpong, R. Total synthesis of (+)-complanadine A using an iridium-catalyzed pyridine C–H functionalization. J. Am. Chem. Soc. 132, 5926–5927 (2010).
Seiple, I. B. et al. Direct C–H arylation of electron-deficient heterocycles with arylboronic acids. J. Am. Chem. Soc. 132, 13194–13196 (2010).
Ye, M. et al. Ligand-promoted C3-selective arylation of pyridines with Pd catalysts: gram-scale synthesis of (±)-preclamol. J. Am. Chem. Soc. 133, 19090–19093 (2011).
Levine, R. & Leake, W. W. Rearrangement in the reaction of 3-bromopyridine with sodium amide and sodioacetophenone. Science 121, 780 (1955).
Zoltewicz, J. A. & Nisi, C. Trapping of 3,4-pyridyne by thiomethoxide ion in ammonia. J. Org. Chem. 34, 765–766 (1969).
May, C. & Moody, C. J. A concise synthesis of the antitumor alkaloid ellipticine. J. Chem. Soc. Chem. Commun. 926–927 (1984).
Gribble, G. W., Saulnier, M. G., Sibi, M. P. & Obaza-Nutaitis, J. A. Synthesis and Diels–Alder reactions of 1,3-dimethyl-4-(phenylsulfonyl)-4H-furo[3,4-b]indole. A new annulation strategy for the construction of ellipticine and isoellipticine. J. Org. Chem. 49, 4518–4523 (1984).
May, C. & Moody, C. J. A new precursor to 3,4-didehydropyridine, and its use in the synthesis of the antitumor alkaloid ellipticine. J. Chem. Soc. Perkin Trans. 1 247–250 (1988).
Díaz, M. T., Cobas, A., Guitián, E. & Castedo, L. Polar control of the regioselectivity of hetaryne cycloadditions. Synthesis of ellipticine. Synlett 157–158 (1998).
Díaz, M. T., Cobas, A., Guitián, E. & Castedo, L. Synthesis of ellipticine by hetaryne cycloadditions – control of regioselectivity. Eur. J. Org. Chem. 4543–4549 (2001).
Enamorado, M. F., Ondachi, P. W. & Comins, D. L. A five-step synthesis of (S)-macrostomine from (S)-nicotine. Org. Lett. 12, 4513–4515 (2010).
Nam, H-H. & Leroi, G. E. First direct observation of pyridyne: matrix infrared study of the photolysis products of 3,4-pyridinedicarboxylic anhydride. J. Am. Chem. Soc. 110, 4096–4097 (1988).
Jamart-Grégoire, B., Leger, C. & Caubère, P. New applications of complex bases: nucleophilic condensations of pyridyne. Tetrahedron Lett. 131, 7599–7602 (1990).
Sha, C-K. & Yang, J-F. Total syntheses of ellipticine alkaloids and their amino analogues. Tetrahedron 48, 10645–10654 (1992).
Tsukazaki, M. & Snieckus, V. Synthetic connections to the directed ortho metalation reaction. 3,4-Pyridynes from 4-trialkylsilyl-3-pyridyl triflates. Heterocycles 33, 533–536 (1992).
Vinter-Pasquier, K., Jamart-Grégoire, B. & Caubère, P. Complex base-induced generation of 3,4-didehydropyridine derivatives: new access to aminopyridines or pyridones. Heterocycles 45, 2113–2129 (1997).
Walters, M. A. & Shay, J. J. 2,3-Pyridyne formation by fluoride-induced desilylation-elimination. Synth. Commun. 27, 3573–3579 (1997).
Carroll, F. I. et al. Synthesis and nicotinic acetylcholine receptor binding properties of bridged and fused ring analogues of epibatidine. J. Med. Chem. 50, 6383–6391 (2007).
Lin, W., Chen, L. & Knochel, P. Preparation of functionalized 3,4-pyridynes via 2-magnesiated diaryl sulfonates. Tetrahedron 63, 2787–2797 (2007).
Jiang, L., Yu, X., Fang, B. & Wu, J. Silver triflate-catalyzed tandem reaction of N′-(2-alkynylbenzylidene)hydrazide with pyridyne. Org. Biomol. Chem. 10, 8102–8107 (2012).
Tadross, P. M. & Stoltz, B. M. A comprehensive history of arynes in natural product total synthesis. Chem. Rev. 112, 3550–3577 (2012).
Reinecke, M. G. Hetarynes. Tetrahedron 38, 427–498 (1982).
Cheong, P. H-Y. et al. Indolyne and aryne distortions and nucleophilic regioselectivities. J. Am. Chem. Soc. 132, 1267–1269 (2010).
Im, G-Y. J. et al. Indolyne experimental and computational studies: synthetic applications and origins of selectivities of nucleophilic additions. J. Am. Chem. Soc. 132, 17933–17944 (2010).
Goetz, A. E. et al. An efficient computational model to predict the synthetic utility of heterocyclic arynes. Angew. Chem. Int. Ed. 51, 2758–2762 (2012).
Spartan 06 (Wavefunction Inc., Irvine, California, 2006).
Bronner, S. M., Goetz, A. E. & Garg, N. K. Overturning indolyne regioselectivities and synthesis of indolactam V. J. Am. Chem. Soc. 133, 3832–3835 (2011).
Rau, N. J. & Wenthold, P. G. Experimental investigation of the absolute enthalpies of formation of 2,3-, 2,4-, and 3,4-pyridynes. J. Phys. Chem. A 115, 10353–10362 (2011).
Himeshima, Y., Sonoda, T. & Kobayashi, H. Fluoride-induced 1,2-elimination of o-trimethylsilylphenyl triflate to benzyne under mild conditions Chem. Lett. 12, 1211–1214 (1983).
Rosen, B. M. et al. Nickel-catalyzed cross-couplings involving carbon–oxygen bonds. Chem. Rev. 111, 1346–1416 (2011).
Quintana, I., Boersma, A. J., Peña, D., Pérez, D., & Guitián, E. Metal-catalyzed cotrimerization of arynes and alkenes. Org. Lett. 8, 3347–3349 (2006).
Gerfaud, T., Neuville, L. & Zhu, J. Palladium-catalyzed annulation of acyloximes with arynes (or alkynes): synthesis of phenanthridines and isoquinolines. Angew. Chem. Int. Ed. 48, 572–577 (2009).
Yoshida, H., Shirakawa, E., Honda, Y. & Hiyama, T. Addition of ureas to arynes: straightforward synthesis of benzodiazepine and benzodiazocine derivatives. Angew. Chem. Int. Ed. 41, 3247–3249 (2002).
Yoshida, H., Fukushima, H., Ohshita, J. & Kunai, A. Arynes in a three-component coupling reaction: straightforward synthesis of benzoannulated iminofurans. Angew. Chem. Int. Ed. 43, 3935–3938 (2004).
Toledo, F. T., Comasseto, J. V. & Raminelli, C. Selenostannylation of arynes produced by silylaryl triflates under mild reaction conditions. J. Braz. Chem. Soc. 21, 2164–2168 (2010).
Effenberger, F. & Daub, W. Darstellung von didehydropyridinen aus (trimethylsilyl)pyridinen. Chem. Ber. 124, 2119–2125 (1991).
Vorbrüggen, H. & Krolikiewicz, K. Silylation-amination of hydroxy N-heterocycles. Chem. Ber. 117, 1523–1541 (1984).
Kaye, H. & Chang, S-H. N-Vinylation of heteroaromatic O-trimethylsilyl lactims. Tetrahedron 26, 1369–1376 (1970).
Liu, Z. & Larock, R. C. Facile N-arylation of amines and sulfonamides and O-arylation of phenols and arenecarboxylic acids. J. Org. Chem. 71, 3198–3209 (2006).
Matsumoto, T., Sohma, T., Hatazaki, S. & Suzuki, K. On the regiochemistry of cycloaddition of unsymmetrical aryne with nitrone. Remarkable effect of trialkylsilyl substituent. Synlett 843–846 (1993).
Dai, M., Wang, Z. & Danishefsky, S. J. A novel α,β-unsaturated nitrone-aryne [3+2] cycloaddition and its application in the synthesis of the cortistatin core. Tetrahedron Lett. 49, 6613–6616 (2008).
Hadjipavlou–Litina, D. & Hansch, C. Quantitative structure–activity relationships of the benzodiazepines. A review and reevaluation. Chem. Rev. 94, 1483–1505 (1994).
Ramgren, S. D., Silberstein, A. L., Yang, Y. & Garg, N. K. Nickel-catalyzed amination of aryl sulfamates. Angew. Chem. Int. Ed. 50, 2171–2173 (2011).
Macklin, T. K. & Snieckus, V. Directed ortho-metalation methodology. The N,N-dialkyl aryl O-sulfamate as a new directed metalation group and cross-coupling partner for Grignard reagents. Org. Lett. 7, 2519–2522 (2005).
Sergeev, A. G. & Hartwig, J. F. Selective, nickel-catalyzed hydrogenolysis of aryl ethers. Science 332, 439–443 (2011).
Mesganaw, T., Fine Nathel, N. F. & Garg, N. K. Cine substitution of arenes using the aryl carbamate as a removable directing group. Org. Lett. 14, 2918–2921 (2012).
Acknowledgements
The authors are grateful to Boehringer Ingelheim, DuPont, Eli Lilly, Amgen, AstraZeneca, Roche, the A. P. Sloan Foundation, the University of California, Los Angeles, the ACS Division of Organic Chemistry (fellowship to A.E.G.) and the Foote Family (fellowship to A.E.G.) for financial support. Jordan Cisneros (University of California, Los Angeles) is acknowledged for experimental assistance and thanks Pfizer for financial support. These studies were supported by shared instrumentation grants from the National Science Foundation (CHE-1048804) and the National Center for Research Resources (S10RR025631).
Author information
Authors and Affiliations
Contributions
A.E.G. planned and carried out the experimental work. A.E.G. and N.K.G. conceived the project, co-wrote the manuscript and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 7373 kb)
Rights and permissions
About this article
Cite this article
Goetz, A., Garg, N. Regioselective reactions of 3,4-pyridynes enabled by the aryne distortion model. Nature Chem 5, 54–60 (2013). https://doi.org/10.1038/nchem.1504
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1504
This article is cited by
-
Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives
Nature (2023)
-
Highly enantioselective catalytic synthesis of chiral pyridines
Nature Communications (2017)
-
Oxime ethers as useful synthons in the synthesis of a number of key medicinal heteroaromatic compounds
Journal of the Iranian Chemical Society (2016)
-
On-surface generation and imaging of arynes by atomic force microscopy
Nature Chemistry (2015)
-
Green strategy from waste to value-added-chemical production: efficient biosynthesis of 6-hydroxy-3-succinoyl-pyridine by an engineered biocatalyst
Scientific Reports (2014)