Abstract
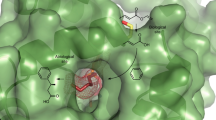
Enzymatic catalysis and homogeneous catalysis offer complementary means to address synthetic challenges, both in chemistry and in biology. Despite its attractiveness, the implementation of concurrent cascade reactions that combine an organometallic catalyst with an enzyme has proven challenging because of the mutual inactivation of both catalysts. To address this, we show that incorporation of a d6-piano stool complex within a host protein affords an artificial transfer hydrogenase (ATHase) that is fully compatible with and complementary to natural enzymes, thus enabling efficient concurrent tandem catalysis. To illustrate the generality of the approach, the ATHase was combined with various NADH-, FAD- and haem-dependent enzymes, resulting in orthogonal redox cascades. Up to three enzymes were integrated in the cascade and combined with the ATHase with a view to achieving (i) a double stereoselective amine deracemization, (ii) a horseradish peroxidase-coupled readout of the transfer hydrogenase activity towards its genetic optimization, (iii) the formation of L-pipecolic acid from L-lysine and (iv) regeneration of NADH to promote a monooxygenase-catalysed oxyfunctionalization reaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wörsdörfer, B., Woycechowsky, K. J. & Hilvert, D. Directed evolution of a protein container. Science 331, 589–592 (2011).
Choudhary, S., Quin, M. B., Sanders, M. A., Johnson, E. T. & Schmidt-Dannert, C. Engineered protein nano-compartments for targeted enzyme localization. PLoS ONE 7, e33342 (2012).
Dueber, J. E. et al. Synthetic protein scaffolds provide modular control over metabolic flux. Nature Biotechnol. 27, 753–759 (2009).
Keasling, J. D. Synthetic biology for synthetic chemistry. ACS Chem. Biol. 3, 64–76 (2008).
Bromley, E. H. C., Channon, K., Moutevelis, E. & Woolfson, D. N. Peptide and protein building blocks for synthetic biology: from programming biomolecules to self-organized biomolecular systems. ACS Chem. Biol. 3, 38–50 (2008).
Weissman, K. J. & Leadlay, P. F. Combinatorial biosynthesis of reduced polyketides. Nature Rev. Microbiol. 3, 925–936 (2005).
Mutti, F. G. et al. Simultaneous iridium catalysed oxidation and enzymatic reduction employing orthogonal reagents. Chem. Commun. 46, 8046–8048 (2010).
Haak, R. M. et al. Dynamic kinetic resolution of racemic β-haloalcohols: direct access to enantioenriched epoxides. J. Am. Chem. Soc. 130, 13508–13509 (2008).
Maid, H. et al. Iron catalysis for in situ regeneration of oxidized cofactors by activation and reduction of molecular oxygen: a synthetic metalloporphyrin as a biomimetic NAD(P)H oxidase. Angew. Chem. Int. Ed. 50, 2397–2400 (2011).
Wasilke, J-C., Obrey, S. J., Baker, R. T. & Bazan, G. C. Concurrent tandem catalysis. Chem. Rev. 105, 1001–1020 (2005).
Zhou, J. Recent advances in multicatalyst promoted asymmetric tandem reactions. Chem. Asian J. 5, 422–434 (2010).
Betanzos-Lara, S. et al. Organometallic ruthenium and iridium transfer-hydrogenation catalysts using coenzyme NADH as a cofactor. Angew. Chem. Int. Ed. 51, 3897–3900 (2012).
Wingstrand, E., Laurell, A., Fransson, L., Hult, K. & Moberg, C. Minor enantiomer recycling: metal catalyst, organocatalyst and biocatalyst working in concert. Chem. Eur. J. 15, 12107–12113 (2009).
Simons, C., Hanefeld, U., Arends, I. W. C. E., Maschmeyer, T. & Sheldon, R. A. Towards catalytic cascade reactions: asymmetric synthesis using combined chemo-enzymatic catalysts. Top. Catal. 40, 35–44 (2006).
Wieczorek, B. Covalent anchoring of a racemization catalyst to CALB-beads: towards dual immobilization of DKR catalysts. Tetrahedron Lett. 52, 1601–1604 (2011).
Rocha-Martín, J., de las Rivas, B., Muñoz, R., Guisán, J. M. & López-Gallego, F. Rational co-immobilization of bi-enzyme cascades on porous supports and their applications in bio-redox reactions with in situ recycling of soluble cofactors. ChemCatChem 4, 1279–1288 (2012).
Hanefeld, U., Gardossi, L. & Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 38, 453–468 (2009).
Brady, D. & Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 31, 1639–1650.
Mateo, C., Palomo, J. M., Fernandez-Lorente, G., Guisan, J. M. & Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Tech. 40, 1451–1463 (2007).
Lopez-Gallego, F. & Schmidt-Dannert, C. Multi-enzymatic synthesis. Curr. Opin. Chem. Biol. 14, 174–183 (2010).
Pàmies, O. & Bäckvall, J-E. Combination of enzymes and metal catalysts. A powerful approach in asymmetric catalysis. Chem. Rev. 103, 3247–3261 (2003).
Kim, Y., Park, J. & Kim, M-J. Dynamic kinetic resolution of amines and amino acids by enzyme–metal cocatalysis. ChemCatChem. 3, 271–277 (2011).
Yusop, R. M., Unciti-Broceta, A., Johansson, E. M. V., Sánchez-Martin, R. M. & Bradley, M. Palladium-mediated intracellular chemistry. Nature Chem. 3, 239–243 (2011).
Foulkes, J. M., Malone, K. J., Coker, V. S., Turner, N. J. & Lloyd, J. R. Engineering a biometallic whole cell catalyst for enantioselective deracemization reactions. ACS Catal. 1, 1589–1594 (2011).
Lu, Y., Yeung, N., Sieracki, N. & Marshall, N. M. Design of functional metalloproteins. Nature 460, 855–862 (2009).
Ward, T. R. Artificial metalloenzymes based on the biotin–avidin technology: enantioselective catalysis and beyond. Acc. Chem. Res. 44, 47–57 (2011).
Bos, J., Fusetti, F., Driessen, A. J. M. & Roelfes, G. Enantioselective artificial metalloenzymes by creation of a novel active site at the protein dimer interface. Angew. Chem. Int. Ed. 51, 7472–7475 (2012).
Jing, Q. & Kazlauskas, R. J. Regioselective hydroformylation of styrene using rhodium-substituted carbonic anhydrase. ChemCatChem 2, 953–957 (2010).
Podtetenieff, J., Taglieber, A., Bill, E., Reijerse, E. J. & Reetz, M. T. An artificial metalloenzyme: creation of a designed copper binding site in a thermostable protein. Angew. Chem. Int. Ed. 49, 5151–5155 (2010).
Deuss, P. J., den Heeten, R., Laan, W. & Kamer, P. C. J. Bioinspired catalyst design and artificial metalloenzymes. Chem. Eur. J. 17, 4680–4698 (2011).
Ueno, T., Abe, S., Yokoi, N. & Watanabe, Y. Coordination design of artificial metalloproteins utilizing protein vacant space. Coord. Chem. Rev. 251, 2717–2731 (2007).
Matsuo, T. et al. Meso-unsubstituted iron corrole in hemoproteins: remarkable differences in effects on peroxidase activities between myoglobin and horseradish peroxidase. J. Am. Chem. Soc. 131, 15124–15125 (2009).
Turner, N. J. Directed evolution drives the next generation of biocatalysts. Nature Chem. Biol. 5, 567–573 (2009).
Turner, N. J. Enantioselective oxidation of C–O and C–N bonds using oxidases. Chem. Rev. 111, 4073–4087 (2011).
Dürrenberger, M. et al. Artificial transfer hydrogenases for the enantioselective reduction of cyclic imines. Angew. Chem. Int. Ed. 50, 3026–3029 (2011).
Rowles, I., Malone, K. J., Etchells, L. L., Willies, S. C. & Turner, N. J. Directed evolution of the enzyme monoamine oxidase (MAO-N): highly efficient chemo-enzymatic deracemisation of the alkaloid (±)-crispine A. ChemCatChem 4, 1259–1261 (2012).
Heiden, Z. M. & Rauchfuss, T. B. Homogeneous catalytic reduction of dioxygen using transfer hydrogenation catalysts. J. Am. Chem. Soc. 129, 14303–14310 (2007).
Brandänge, S., Lindblom, L., Pilotti, Å. & Rodriguez, B. Ring-chain tautomerism of pseudooxynicotine and some other iminium compounds. Acta Chem. Scand. B 37, 617–622 (1983).
Truppo, M. D., Escalettes, F. & Turner, N. J. Rapid determination of both the activity and enantioselectivity of ketoreductases. Angew. Chem. Int. Ed. 47, 2639–2641 (2008).
Yasuda, M., Ueda, M., Muramatsu, H., Mihara, H. & Esaki, N. Enzymatic synthesis of cyclic amino acids by N-methyl-L-amino acid dehydrogenase from Pseudomonas putida. Tetrahedron Asymm. 17, 1775–1779 (2006).
Gatto, G. J., Boyne, M. T., Kelleher, N. L. & Walsh, C. T. Biosynthesis of pipecolic acid by RapL, a lysine cyclodeaminase encoded in the rapamycin gene cluster. J. Am. Chem. Soc. 128, 3838–3847 (2006).
Hollmann, F., Hofstetter, K. & Schmid, A. Non-enzymatic regeneration of nicotinamide and flavin cofactors for monooxygenase catalysis. Trends Biotechnol. 24, 163–171 (2006).
Hollmann, F., Arends, I. W. C. E. & Buehler, K. Biocatalytic redox reactions for organic synthesis: nonconventional regeneration methods. ChemCatChem 2, 762–782 (2010).
Poizat, M., Arends, I. W. C. E. & Hollmann, F. On the nature of mutual inactivation between [Cp*Rh(bpy)(H2O)]2+ and enzymes — analysis and potential remedies. J. Mol. Catal. B 63, 149–156 (2010).
Hildebrand, F. & Lütz, S. Stable electroenzymatic processes by catalyst separation. Chem. Eur. J. 15, 4998–5001 (2009).
Haquette, P. et al. Chemically engineered papain as artificial formate dehydrogenase for NAD(P)H regeneration. Org. Biomol. Chem. 9, 5720–5727 (2011).
Maenaka, Y., Suenobu, T. & Fukuzumi, S. Efficient catalytic interconversion between NADH and NAD+ accompanied by generation and consumption of hydrogen with a water-soluble iridium complex at ambient pressure and temperature. J. Am. Chem. Soc. 134, 367–374 (2012).
Canivet, J., Süss-Fink, G. & Štěpnička, P. Water-soluble phenanthroline complexes of rhodium, iridium and ruthenium for the regeneration of NADH in the enzymatic reduction of ketones. Eur. J. Inorg. Chem. 4736–4742 (2007).
Ryan, J. D., Fish, R. H. & Clark, D. S. Engineering cytochrome P450 enzymes for improved activity towards biomimetic 1,4-NADH cofactors. ChemBioChem 9, 2579–2582 (2008).
Acknowledgements
This work was supported by the Marie Curie Initial Training Network (Biotrains FP7-ITN-238531). T.R.W. acknowledges financial support from the SNF (Schweizerische Nationalfonds, grant no. 200020_126366) and the National Centre of Competence in Research Nanosciences. N.J.T. acknowledges the Royal Society for a Wolfson Research Merit Award. F.H. thanks A. Schmid (Dortmund University of Technology) for the kind provision of HbpA. The authors also thank M. Corbett, S. Willies and K. Malone for helpful advice and materials, R. Pfalzberger for help with the graphic material, and Umicore for a precious metal loan.
Author information
Authors and Affiliations
Contributions
V.K., F.H., N.T. and T.W. conceived the catalytic cascades. V.K., F.H., N.T. and T.W. supervised the project. V.K., Y.W., M.D., D.G., E.C. and T.Q. performed the experiments. V.K., Y.W., M.D., D.G., E.C., T.Q., F.H., N.T. and T.W. analysed the data. V.K., F.H., N.T. and T.W. co-wrote the paper. V.K., Y.W., M.D., D.G. and L.K. contributed materials. D.H. analysed the conversion of 13C-labelled lysine by 2D NMR.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3026 kb)
Rights and permissions
About this article
Cite this article
Köhler, V., Wilson, Y., Dürrenberger, M. et al. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes. Nature Chem 5, 93–99 (2013). https://doi.org/10.1038/nchem.1498
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1498
This article is cited by
-
Artificially sporulated Escherichia coli cells as a robust cell factory for interfacial biocatalysis
Nature Communications (2022)
-
An artificial metalloenzyme biosensor can detect ethylene gas in fruits and Arabidopsis leaves
Nature Communications (2019)
-
Biocompatibility and therapeutic potential of glycosylated albumin artificial metalloenzymes
Nature Catalysis (2019)
-
Chemo-enzymatic cascades to produce cycloalkenes from bio-based resources
Nature Communications (2019)
-
Biochemical Characteristics of Microbial Enzymes and Their Significance from Industrial Perspectives
Molecular Biotechnology (2019)