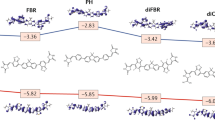
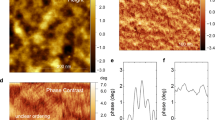
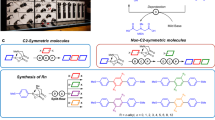
Abstract
Organic semiconducting materials based on polymers and molecular systems containing an electronically delocalized structure are the basis of emerging optoelectronic technologies such as plastic solar cells and flexible transistors. For isolated molecules, guidelines exist that rely on the molecular formula to tailor the frontier (highest occupied or lowest unoccupied) molecular orbital energy levels and optical absorption profiles. Much less control can be achieved over relevant properties, however, as one makes the transition to the ensemble behaviour characteristic of the solid state. Polymeric materials are also challenging owing to the statistical description of the average number of repeat units. Here we draw attention to the limitations of molecular formulae as predictive tools for achieving properties relevant to device performances. Illustrative examples highlight the relevance of organization across multiple length scales, and how device performances — although relevant for practical applications — poorly reflect the success of molecular design.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nozik, A. J. & Miller, J. Introduction to solar photon conversion. Chem. Rev. 110, 6443–6445 (2010).
Mishra, A. & Bäuerle, P. Small molecule organic semiconductors on the move: Promises for future solar energy technology. Angew. Chem. Int. Ed. 51, 2020–2067 (2012).
Roncali, J. Molecular bulk heterojunctions: An emerging approach to organic solar cells. Acc. Chem. Res. 42, 1719–1730 (2009).
Cheng, Y. J., Yang, S. H. & Hsu, C. S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 109, 5868–5923 (2009).
Beaujuge, P. M. & Fréchet, J. M. J. Molecular design and ordering effects in π-functional materials for transistor and solar cell applications. J. Am. Chem. Soc. 133, 20009–20029 (2011).
Gendron, D. & Leclerc, M. New conjugated polymers for organic solar cells. Energy Environ. Sci. 4, 1225–1237 (2011).
Zhou, H., Yang, L. & You, W. Rational design of high performance conjugated polymers for organic solar cells. Macromolecules 45, 607–632 (2012).
Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 23, 733–758 (2010).
Tsao, H. N. et al. Ultrahigh mobility in polymer field-effect transistors by design. J. Am. Chem. Soc. 133, 2605–2612 (2011).
Leong, W. L. et al. Role of trace impurities in the photovoltaic performance of solution processed small-molecule bulk heterojunction solar cells. Chem. Sci. 3, 2103–2109 (2012).
Nielsen, K. T., Bechgaard, K. & Krebs, F. C. Removal of palladium nanoparticles from polymer materials. Macromolecules 38, 658–659 (2005).
Wang, S., Kiersnowski, A., Pisula, W. & Müllen, K. Microstructure evolution and device performance in solution-processed polymeric field-effect transistors: The Key role of the first monolayer. J. Am. Chem. Soc. 134, 4015–4018 (2012).
Peet, J. et al. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nature Mater. 6, 497–500 (2007).
Steim, R., Kogler, F. R. & Brabec, C. J. Interface materials for organic solar cells. J. Mater. Chem. 20, 2499–2512 (2010).
Hoeben, F. J. M., Jonkheijm, P., Meijer, E. W. & Schenning, A. P. H. J. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 105, 1491–1546 (2005).
Giridharagopal, R. & Ginger, D. S. Characterizing morphology in bulk heterojunction organic photovoltaic systems. J. Phys. Chem. Lett. 1, 1160–1169 (2010).
Coffin, R. C., Peet, J., Rogers, J. & Bazan, G. C. Streamlined microwave-assisted preparation of narrow-bandgap conjugated polymers for high-performance bulk heterojunction solar cells. Nature Chem. 1, 657–661 (2009).
Okamoto, K. & Luscombe, C. K. Controlled polymerizations for the synthesis of semiconducting conjugated polymers. Polym. Chem. 2, 2424–2434 (2011).
Carsten, B., He, F., Son, H. J., Xu, T. & Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 111, 1493–1528 (2011).
Berrouard, P. et al. Synthesis of 5-alkyl[3,4-c]thienopyrrole-4,6-dione-based polymers by direct heteroarylation. Angew. Chem. Int. Ed. 51, 2068–2071 (2012).
Osaka, I. & McCullough, R. D. Advances in molecular design and synthesis of regioregular polythiophenes. Acc. Chem. Res. 41, 1202–1214 (2008).
Kline, R. J., McGehee, M. D., Kadnikova, E. N., Liu, J. & Fréchet, J. M. J. Controlling the field-effect mobility of regioregular polythiophene by changing the molecular weight. Adv. Mater. 15, 1519–1522 (2003).
Jeffries-El, M., Sauvé, G. & McCullough, R. D. In-situ end-group functionalization of regioregular poly(3-alkylthiophene) using the Grignard metathesis polymerization method. Adv. Mater. 16, 1017–1019 (2004).
Kim, Y. et al. Effect of the end group of regioregular poly(3-hexylthiophene) polymers on the performance of polymer/fullerene solar cells. J. Phys. Chem. C 111, 8137–8141 (2007).
Park, J. K. et al. End-capping effect of a narrow bandgap conjugated polymer on bulk heterojunction solar cells. Adv. Mater. 23, 2430–2435 (2011).
Kim, J. S. et al. High-efficiency organic solar cells based on end-functional-group-modified poly(3-hexylthiophene). Adv. Mater. 22, 1355–1360 (2010).
Sirringhaus, H. et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 401, 685–688 (1999).
Kim, Y. et al. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene:fullerene solar cells. Nature Mater. 5, 197–203 (2006).
Ying, L. et al. Regioregular pyridal[2,1,3]thiadiazole π-conjugated copolymers. J. Am. Chem. Soc. 133, 18538–18541 (2011).
McCulloch, I. et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nature Mater. 5, 328–333 (2006).
Chabinyc, M. L., Toney, M. F., Kline, R. J., McCulloch, I. & Heeney, M. X-ray scattering study of thin films of poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]thiophene). J. Am. Chem. Soc. 129, 3226–3237 (2007).
Gidron, O., Diskin-Posner, Y. & Bendikov, M. α-Oligofurans. J. Am. Chem. Soc. 132, 2148–2150 (2010).
Patra, A. & Bendikov, M. Polyselenophenes. J. Mater. Chem. 20, 422–433 (2010).
Jahnke, A. A. & Seferos, D. S. Polytellurophenes. Macromol. Rapid Commun. 32, 943–951 (2011).
Chen, H. Y. et al. Silicon atom substitution enhances interchain packing in a thiophene-based polymer system. Adv. Mater. 22, 371–375 (2010).
Scharber, M. C. et al. Influence of the bridging atom on the performance of a low-bandgap bulk heterojunction solar cell. Adv. Mater. 22, 367–370 (2010).
Amb, C. M. et al. Dithienogermole as a fused electron donor in bulk heterojunction solar cells. J. Am. Chem. Soc. 133, 10062–10065 (2011).
Dang, M. T., Hirsch, L. & Wantz, G. P3HT:PCBM, best seller in polymer photovoltaic research. Adv. Mater. 23, 3597–3602 (2011).
Piliego, C. et al. Synthetic control of structural order in N-alkylthieno[3,4-c]pyrrole-4,6-dione-based polymers for efficient solar cells. J. Am. Chem. Soc. 132, 7595–7597 (2010).
Zou, Y. et al. A thieno[3,4-c]pyrrole-4,6-dione-based copolymer for efficient solar cells. J. Am. Chem. Soc. 132, 5330–5331 (2010).
Zhang, Y. et al. Efficient polymer solar cells based on the copolymers of benzodithiophene and thienopyrroledione. Chem. Mater. 22, 2696–2698 (2010).
Peet, J., Senatore, M. L., Heeger, A. J. & Bazan, G. C. The role of processing in the fabrication and optimization of plastic solar cells. Adv. Mater. 21, 1521–1527 (2009).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Würthner, F. & Meerholz, K. Systems chemistry approach in organic photovoltaics. Chem. Eur. J. 16, 9366–9373 (2010).
Sun, Y. et al. Solution-processed small-molecule solar cells with 6.7% efficiency. Nature Mater. 11, 44–48 (2012).
Henson, Z. B., Welch, G. C., van der Poll, T. & Bazan, G. C. Pyridalthiadiazole-based narrow band gap chromophores. J. Am. Chem. Soc. 134, 3766–3779 (2012).
Fitzner, R. et al. Interrelation between crystal packing and small-molecule organic solar cell performance. Adv. Mater. 24, 675–680 (2012).
Tong, M. et al. Higher molecular weight leads to improved photoresponsivity, charge transport and interfacial ordering in a narrow bandgap semiconducting polymer. Adv. Funct. Mater. 20, 3959–3965 (2010).
Herzing, A. A., Richter, L. J. & Anderson, I. M. 3D nanoscale characterization of thin-film organic photovoltaic device structures via spectroscopic contrast in the TEM 1. J. Phys. Chem. C 114, 17501–17508 (2010).
Hammond, M. R. et al. Molecular order in high-efficiency polymer/fullerene bulk heterojunction solar cells. ACS Nano 5, 8248–8257 (2011).
Acknowledgements
Work done at UCSB has been supported through the NSF (DMR 1005546). We gratefully thank Peter Allen for assistance with image preparation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Henson, Z., Müllen, K. & Bazan, G. Design strategies for organic semiconductors beyond the molecular formula. Nature Chem 4, 699–704 (2012). https://doi.org/10.1038/nchem.1422
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1422
This article is cited by
-
π-Extended perylene diimide double-heterohelicenes as ambipolar organic semiconductors for broadband circularly polarized light detection
Nature Communications (2021)
-
Dodecyl-substituted poly(3,4-ethylenedioxyselenophene): polymerization and its solution-processable applications for electrochromic and organic solar cells
Journal of Polymer Research (2021)
-
Insight into the structures and dynamics of organic semiconductors through solid-state NMR spectroscopy
Nature Reviews Materials (2020)
-
Synthesis, optical limiting behavior, thermal blooming and nonlinear studies of dye-doped polymer films
Journal of Materials Science: Materials in Electronics (2020)
-
Strong modification of the transport level alignment in organic materials after optical excitation
Nature Communications (2019)