Abstract
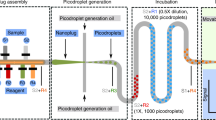
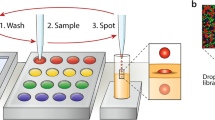
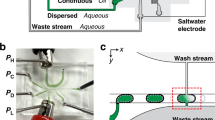
Pipetting and dilution are universal processes used in chemical and biological laboratories to assay and experiment. In microfluidics such operations are equally in demand, but difficult to implement. Recently, droplet-based microfluidics has emerged as an exciting new platform for high-throughput experimentation. However, it is challenging to vary the concentration of droplets rapidly and controllably. To this end, we developed a dilution module for high-throughput screening using droplet-based microfluidics. Briefly, a nanolitre-sized sample droplet of defined concentration is trapped within a microfluidic chamber. Through a process of droplet merging, mixing and re-splitting, this droplet is combined with a series of smaller buffer droplets to generate a sequence of output droplets that define a digital concentration gradient. Importantly, the formed droplets can be merged with other reagent droplets to enable rapid chemical and biological screens. As a proof of concept, we used the dilutor to perform a high-throughput homogeneous DNA-binding assay using only nanolitres of sample.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hertzberg, R. P. & Pope, A. J. High-throughput screening: new technology for the 21st century. Curr. Opin. Chem. Biol. 4, 445–451 (2000).
Sundberg, S. A. High-throughput and ultra-high-throughput screening: solution- and cell-based approaches. Curr. Opin. Biotechnol. 11, 47–53 (2000).
Xia, Y. N. & Whitesides, G. M. Soft lithography. Ann. Rev. Mater. Sci. 28, 153–184 (1998).
Unger, M. A., Chou, H. P., Thorsen, T., Scherer, A. & Quake, S. R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116 (2000).
deMello, A. J. Control and detection of chemical reactions in microfluidic systems. Nature 442, 394–402 (2006).
Thorsen, T., Maerkl, S. J. & Quake, S. R. Microfluidic large-scale integration. Science 298, 580–584 (2002).
Gomez-Sjoberg, R., Leyrat, A. A., Pirone, D. M., Chen, C. S. & Quake, S. R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 79, 8557–8563 (2007).
Hansen, C. L., Skordalakes, E., Berger, J. M. & Quake, S. R. A robust and scalable microfluidic metering method that allows protein crystal growth by free interface diffusion. Proc. Natl Acad. Sci. USA 99, 16531–16536 (2002).
Jeon, N. L. et al. Generation of solution and surface gradients using microfluidic systems. Langmuir 16, 8311–8316 (2000).
Dertinger, S. K. W., Chiu, D. T., Jeon, N. L. & Whitesides, G. M. Generation of gradients having complex shapes using microfluidic networks. Anal. Chem. 73, 1240–1246 (2001).
Chung, B. G. et al. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 5, 401–406 (2005).
Stone, H. A., Stroock, A. D. & Ajdari, A. Engineering flows in small devices: microfluidics toward a lab-on-a-chip. Ann. Rev. Fluid Mech. 36, 381–411 (2004).
Makamba, H., Kim, J. H., Lim, K., Park, N. & Hahn, J. H. Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis 24, 3607–3619 (2003).
Song, H., Chen, D. L. & Ismagilov, R. F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. 45, 7336–7356 (2006).
Thorsen, T., Roberts, R. W., Arnold, F. H. & Quake, S. R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 86, 4163–4166 (2001).
Anna, S. L., Bontoux, N. & Stone, H. A. Formation of dispersions using ‘flow focusing’ in microchannels. Appl. Phys. Lett. 82, 364–366 (2003).
Song, H. & Ismagilov, R. F. Millisecond kinetics on a microfluidic chip using nanoliters of reagents. J. Am. Chem. Soc. 125, 14613–14619 (2003).
Brouzes, E. et al. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl Acad. Sci. USA 106, 14195–14200 (2009).
Link, D. R. et al. Electric control of droplets in microfluidic devices. Angew. Chem. Int. Ed. 45, 2556–2560 (2006).
Prakash, M. & Gershenfeld, N. Microfluidic bubble logic. Science 315, 832–835 (2007).
Niu, X., Gulati, S., Edel, J. B. & deMello, A. J. Pillar-induced droplet merging in microfluidic circuits. Lab Chip 8, 1837–1841 (2008).
Niu, X. Z., Gielen, F., deMello, A. J. & Edel, J. B. Electro-coalescence of digitally controlled droplets. Anal. Chem. 81, 7321–7325 (2009).
Tan, Y. C., Fisher, J. S., Lee, A. I., Cristini, V. & Lee, A. P. Design of microfluidic channel geometries for the control of droplet volume, chemical concentration, and sorting. Lab Chip 4, 292–298 (2004).
Niu, X. Z. et al. Droplet-based compartmentalization of chemically separated components in two-dimensional separations. Chem. Commun. 6159–6161 (2009).
Garstecki, P., Fuerstman, M. J., Stone, H. A. & Whitesides, G. M. Formation of droplets and bubbles in a microfluidic T-junction – scaling and mechanism of break-up. Lab Chip 6, 437–446 (2006).
Bremond, N., Thiam, A. R. & Bibette, J. Decompressing emulsion droplets favors coalescence. Phys. Rev. Lett. 100, 024501 (2008).
Shi, W. W., Qin, J. H., Ye, N. N. & Lin, B. C. Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab on a Chip 8, 1432–1435 (2008).
Fidalgo, L. M., Abell, C. & Huck, W. T. S. Surface-induced droplet fusion in microfluidic devices. Lab Chip 7, 984–986 (2007).
Ottino, J. M. The Kinematics of Mixing: Stretching, Chaos, and Transport (Cambridge Univ. Press, 1989).
Tice, J. D., Song, H., Lyon, A. D. & Ismagilov, R. F. Formation of droplets and mixing in multiphase microfluidics at low values of the Reynolds and the capillary numbers. Langmuir 19, 9127–9133 (2003).
Srisa-Art, M., deMello, A. J. & Edel, J. B. High-throughput DNA droplet assays using picoliter reactor volumes. Anal. Chem. 79, 6682–6689 (2007).
Acknowledgements
This work was partially supported by the Research Councils UK Basic Technology Programme (Grant EP/D048664/1) and the National Research Foundation of Korea (Grant Number R11-2009-044-1002-0K20904000004-09A050000410).
Author information
Authors and Affiliations
Contributions
X.N. conceived the dilution module, X.N., F.G., J.B.E. and A.J.D. designed the experiments, X.N. and F.G. performed the experiments, X.N., J.B.E. and F.G. analysed the data, and X.N. and A.J.D. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 17338 kb)
Supplementary information
Supplementary Movie S1 (AVI 27764 kb)
Rights and permissions
About this article
Cite this article
Niu, X., Gielen, F., Edel, J. et al. A microdroplet dilutor for high-throughput screening. Nature Chem 3, 437–442 (2011). https://doi.org/10.1038/nchem.1046
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1046
This article is cited by
-
A general strategy for high-throughput experimental screening of promising bulk thermoelectric materials
Science China Materials (2021)
-
Droplet-based optofluidic systems for measuring enzyme kinetics
Analytical and Bioanalytical Chemistry (2020)
-
Monitoring biomolecule concentrations in tissue using a wearable droplet microfluidic-based sensor
Nature Communications (2019)
-
Study of droplet flow in a T-shape microchannel with bottom wall fluctuation
Acta Mechanica Sinica (2018)
-
Effects of geometry factors on microvortices evolution in confined square microcavities
Microfluidics and Nanofluidics (2018)