Abstract
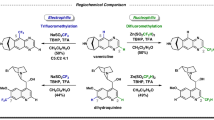
Carbon–carbon bond formation is the basis for the biogenesis of nature's essential molecules. Consequently, it lies at the heart of the chemical sciences. Chiral catalysts have been developed for asymmetric C–C bond formation to yield single enantiomers from several organometallic reagents. Remarkably, for extremely reactive organolithium compounds, which are among the most broadly used reagents in chemical synthesis, a general catalytic methodology for enantioselective C–C formation has proven elusive, until now. Here, we report a copper-based chiral catalytic system that allows carbon–carbon bond formation via allylic alkylation with alkyllithium reagents, with extremely high enantioselectivities and able to tolerate several functional groups. We have found that both the solvent used and the structure of the active chiral catalyst are the most critical factors in achieving successful asymmetric catalysis with alkyllithium reagents. The active form of the chiral catalyst has been identified through spectroscopic studies as a diphosphine copper monoalkyl species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
15 March 2011
In the version of this Article originally published, the Acknowledgements section was incorrect. This error has now been corrected in all versions of the Article.
References
Tidwell, T. T. Wilhelm Schlenk: the man behind the flask. Angew. Chem. Int. Ed. 40, 331–337 (2001).
Schlenk, W. & Holtz, J. The simplest organometallic alkali compounds. Ber. Dtsch. Chem. Ges. 50, 262–274 (1917).
Rappoport, Z. & Marek, I. (eds) The Chemistry of Organolithium Compounds. (Wiley-VCH, 2004).
Nájera, C. & Yus, M. Functionalized organolithium compounds: new synthetic adventures. Curr. Org. Chem. 7, 867–926 (2003).
Hodgson, D. M. (eds) Organolithiums in Enantioselective Synthesis. (Springer-Verlag, 2003).
Majewski, M. & Snieckus, V. (eds) Organometallics: Compounds of Group 1 (Li…Cs). (Thieme, 2006).
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (eds) Comprehensive Asymmetric Catalysis: Suppl. 2. (Springer-Verlag, 2004).
Noyori, R. (eds) Asymmetric Catalysis in Organic Synthesis. (John Wiley & Sons, 1994).
Teichert, J. F. & Feringa, B. L. Phosphoramidites: privileged ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 49, 2486–2528 (2010).
Feringa, B. L., Naasz, R., Imbos, R. & Arnold, L. A. Copper-catalyzed enantioselective conjugate addition reactions of organozinc reagents, in Modern Organocopper Chemistry (ed. Krause, N.) 224–258 (Wiley-VCH, 2002).
Hird, A. W. & Hoveyda, A. H. Catalytic enantioselective alkylations of tetrasubstituted olefins. Synthesis of all-carbon quaternary stereogenic centers through Cu-catalyzed asymmetric conjugate additions of alkylzinc reagents to enones. J. Am. Chem. Soc. 127, 14988–14989 (2005).
Alexakis, A., Bäckvall, J. E., Krause, N., Pàmies, O. & Diéguez, M. Enantioselective copper-catalyzed conjugate addition and allylic substitution reactions. Chem. Rev. 108, 2796–2823 (2008).
Gao, F., McGrath, K. P., Lee, Y., Mandai, K., Hoveyda, A. H. Synthesis of quaternary carbon stereogenic centers through enantioselective Cu-catalyzed allylic substitutions with vinyaluminum reagents. J. Am. Chem. Soc. 132, 14315–14320 (2010).
Hawner, C., Cirriez, V., Li, K. & Alexakis, A. Copper-catalyzed asymmetric conjugate addition of aryl aluminum reagents to trisubstituted enones: construction of aryl-substituted quaternary centers. Angew. Chem. Int. Ed. 47, 8211–8214 (2008).
Gao, F., Lee, Y., Mandai, K. & Hoveyda, A. H. Quaternary carbon stereogenic centers through copper-catalyzed enantioselective allylic substitutions with readily accessible aryl- or hetero-aryllithium reagents and aluminum chlorides. Angew. Chem. Int. Ed. 122, 8548–8552 (2010).
Feringa, B. L., Badorrey, R., Peña, D., Harutyunyan, S. R. & Minnaard, A. J. Copper-catalyzed asymmetric conjugate addition of Grignard reagents to cyclic enones Proc. Natl Acad. Sci. USA 101, 5834–5838 (2004).
Harutyunyan, S. R., den Hartog, T., Geurts, K., Minnaard, A. J. & Feringa, B. L. Catalytic asymmetric conjugate addition and allylic alkylation with Grignard reagents. Chem. Rev. 108, 2824–2852 (2008).
Thaler, T. & Knochel, P. Copper-catalyzed asymmetric Michael addition of magnesium, zinc, and aluminum organometallic reagents: efficient synthesis of chiral molecules. Angew. Chem. Int. Ed. 48, 645–648 (2009).
Corey, E. J., Naef, R. & Hannon, F. J. Enantioselective conjugate addition of rationally designed chiral cuprate reagents to 2-cycloalkenones J. Am. Chem. Soc. 108, 7114–7116. (1986).
Denmark, S. E. & Stiff, C. M. Effect of ligand structure in the bisoxazoline mediated asymmetric addition of methyllithium to imines. J. Org. Chem. 65, 5875–5878 (2000).
Tanaka, K., Matsui, J. & Suzuki, H. Chiral amplification and the catalytic process in the enantioselective conjugate addition of chiral alkoxydimethylcuprate to (E)-cyclopentadec-2-en-1-one. J. Chem. Soc. Perkin Trans. 1, 153–157 (1993).
Beng, T. K. & Gawley, R. E. Highly enantioselective catalytic dynamic resolution of N-Boc-2-lithiopiperidine: synthesis of (R)-(+)-N-Boc-pipecolic acid, (S)-(–)-coniine, (S)-(+)-pelletierine, (+)-conhydrine, and (S)-(–)-ropivacaine and formal synthesis of (–)-lasubine II and (+)-cermizine C. J. Am. Chem. Soc. 132, 12216–12217 (2010).
Bilke, J. L., Moore, S. P., O'Brien, P. & Gilday, J. Catalytic asymmetric synthesis of piperidines from pyrrolidine: concise synthesis of L-733,060. Org. Lett. 11, 1935–1938 (2009).
Tomioka, K., Inoue, I., Mitsuru, I., Kenji, S. & Koga, K. Catalytic asymmetric addition of organolithiums to aldimines. Tetrahedron Lett. 24, 3095–3098 (1991).
Inoue, I., Mitsuru, I., Kenji, S., Koga, K. & Tomioka, K. Asymmetric 1,2-addition of organolithiums to aldimines catalyzed by chiral ligand. Tetrahedron 50, 4429–4438 (1994).
Alexakis, A. & Amiot, F. Enantioselective addition of organolithium reagents on isoquinoline. Tetrahedron: Asymmetry 13, 2117–2122 (2002).
Denmark, S. E., Nakajima, N. & Nicaise, O. J.-C. Asymmetric addition of organolithium reagents to imines. J. Am. Chem. Soc. 116, 8797–8798 (1994).
Denmark, S. E. & Nicaise, O. J.-C. Ligand-mediated addition of organometallic reagents to azomethine functions. Chem. Commun. 999–1004 (1996).
Luderer, M. R. et al. Asymmetric addition of achiral organomagnesium reagents or organolithiums to achiral aldehydes or ketones: a review. Tetrahedron: Asymmetry 20, 981–998 (2009).
Gessner, V. H., Däschlein, C. & Strohmann, C. Structure formation principles and reactivity of organolithium compounds. Chem. Eur. J. 15, 3320–3334 (2009).
Paquin, J.-F. & Lautens, M. Allylic substitution reactions, in Comprehensive Asymmetric Catalysis: Suppl. 2 (eds, Jacobsen, E. N., Pfaltz, A. & Yamamoto, H.) 73–95 (Springer-Verlag, 2004).
Dai, L.-X. & Hou, X.-L. (eds) Chiral Ferrocene Ligands in Asymmetric Catalysis (Wiley-VCH, 2010).
van Zijl, A. W., Arnold, L. A., Minnaard, A. J. & Feringa, B. L. Highly enantioselective copper-catalyzed allylic alkylation with phosphoramidite ligands. Adv. Synth. Catal. 346, 413–420 (2004).
Alexakis, A. & Croset, K. Tandem copper-catalyzed enantioselective allylation-metathesis Org. Lett. 4, 4147–4149 (2002).
Van Veldhuizen, J. J., Campbell, J. E., Giudici, R. E. & Hoveyda, A. H. A readily available chiral Ag-based N-heterocyclic carbene complex for use in efficient and highly enantioselective Ru-catalyzed olefin metathesis and Cu-catalyzed allylic alkylation reactions. J. Am. Chem. Soc. 127, 6877–6882 (2005).
Geurts, K., Fletcher, S. P. & Feringa, B. L. Copper catalyzed asymmetric synthesis of chiral allylic esters J. Am. Chem. Soc. 128, 15572–15573 (2006).
Harutyunyan, S. R. et al. On the mechanism of the copper-catalyzed enantioselective 1,4-addition of Grignard reagents to α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 128, 9103–9118 (2006).
Acknowledgements
The authors acknowledge the Netherlands Organization for Scientific Research (NWO-CW) and the National Research School Catalysis (NRSC-C) for financial support. M.P. thanks the Xunta de Galicia for an Angeles Alvariño contract and Fondo Social Europeo. M.F.-M. thanks the Spanish Ministry of Science and Innovation (MICINN) for a postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
M.P. and M.F.-M. studied solvent effects and optimized the reaction conditions. P.H.B. performed ligand screening. A.R. performed copper salt screening. S.R.H. carried out NMR studies. M.P., M.F.-M, P.H.B. and A.R. evaluated the scope of the organolithium addition reaction. All authors contributed to designing the experiments, analysing the data and editing the manuscript. S.R.H. and B.L.F. guided the research and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1439 kb)
Rights and permissions
About this article
Cite this article
Pérez, M., Fañanás-Mastral, M., Bos, P. et al. Catalytic asymmetric carbon–carbon bond formation via allylic alkylations with organolithium compounds. Nature Chem 3, 377–381 (2011). https://doi.org/10.1038/nchem.1009
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1009
This article is cited by
-
Decarboxylative olefination of potassium benzoates via bimetallic catalysis strategy
Monatshefte für Chemie - Chemical Monthly (2018)
-
Cu-catalyzed enantioselective allylic alkylation with organolithium reagents
Nature Protocols (2017)
-
Direct catalytic cross-coupling of organolithium compounds
Nature Chemistry (2013)
-
Catalytic asymmetric carbon–carbon bond formation using alkenes as alkylmetal equivalents
Nature Chemistry (2012)