Abstract
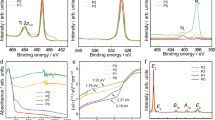
Titanium dioxide is the prototypical transition metal oxide photocatalyst. However, the larger than 3 eV bandgap of common bulk phases of TiO2 limits its light absorption to UV light, making it inefficient for solar energy conversion. Attempts at increasing visible light activity by narrowing the bandgap of TiO2 through doping have proven difficult, because of defect-induced charge trapping and recombination sites of photo-excited charge carriers. Here, we report the existence of a dopant-free, pure TiO2 phase with a narrow bandgap. This new pure TiO2 phase forms on the surface of rutile TiO2(011) by oxidation of bulk titanium interstitials. We measure a bandgap of only ~2.1 eV for this new phase, matching it closely with the energy of visible light.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Fujishima, A., Zhang, X. & Tryk, D. A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 63, 515–582 (2008).
Choi, W., Termin, A. & Hoffmann, M. R. The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 98, 13669–13679 (1994).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001).
Khan, S. U. M., Al-Shahry, M. & Ingler, W. B. Jr. Efficient photochemical water splitting by a chemically modified n-TiO2 . Science 297, 2243–2245 (2002).
Kim, D. Y., Almeida, J. S. d., KoCi, L. & Ahuja, R. Dynamical stability of the hardest known oxide and the cubic solar material: TiO2 . Appl. Phys. Lett. 90, 171903 (2007).
Mattesini, M. et al. Cubic TiO2 as a potential light absorber in solar-energy conversion. Phys. Rev. B 70, 115101 (2004).
Ariga, H. et al. Surface-mediated visible-light photo-oxidation on pure TiO2(001). J. Am. Chem. Soc. 131, 14670–14672 (2009).
Papageorgiou A. C. et al. Electron traps and their effect on the surface chemistry of TiO2(110). Proc. Natl Acad. Sci. USA 107, 2391–2396 (2010).
Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003).
Torrelles, X. et al. Geometric structure of TiO2(011)(2×1). Phys. Rev. Lett. 101, 185501 (2008).
Gong, X.-Q. et al. The 2×1 reconstruction of the rutile TiO2(011) surface: a combined density functional theory, X-ray diffraction, and scanning tunneling microscopy study. Surf. Sci. 603, 138–144 (2009).
Li, M. et al. Oxygen-induced restructuring of the TiO2(110) surface: a comprehensive study. Surf. Sci. 437, 173–190 (1999).
Bowker, M. & Bennett, R. A. The role of Ti3+ interstitials in TiO2 (110) reduction and oxidation. J. Phys. Condens. Matter 21, 474224 (2009).
Stone, P., Bennett, R. A. & Bowker, M. Reactive re-oxidation of reduced TiO2 (110) surfaces demonstrated by high temperature STM movies. New J. Phys. 1, 8 (1999).
McCarty, K. F. Growth regimes of the oxygen-deficient TiO2(110) surface exposed to oxygen. Surf. Sci. 543, 185–206 (2003).
Dulub, O., Valentin, C. D., Selloni, A. & Diebold, U. Structure, defects, and impurities at the rutile TiO2(011)–(2×1) surface: a scanning tunneling microscopy study. Surf. Sci. 600, 4407–4417 (2006).
Batzill, M., Katsiev, K., Gaspar, D. J. & Diebold, U. Variations of the local electronic surface properties of TiO2(110) induced by intrinsic and extrinsic defects. Phys. Rev. B 66, 235401 (2002).
Thomas, A. G. et al. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and X-ray absorption spectroscopy. Phys. Rev. B 75, 035105 (2007).
Zhang, Z., Jeng, S.-P. & Henrich, V. E. Cation–ligand hybridization for stoichiometric and reduced TiO2 (110) surfaces determined by resonant photoemission. Phys. Rev. B 43, 12004–12011 (1991).
Henrich, V. E., Dresselhaus, G. & Zeiger, H. J. Observation of two-dimensional phases associated with defect states on the surface of TiO2 . Phys. Rev. Lett. 36, 1335–1338 (1976).
Fano, U. Effects of configuration interaction on intensities and phase shifts. Phys. Rev. 124, 1866–1878 (1961).
Smith, K. E. & Henrich, V. E. Resonant photoemission in Ti2O3 and V2O3: hybridization and localization of cation 3d orbitals. Phys. Rev. B 38, 9571–9580 (1988).
Hebenstreit, E. L. D. et al. Sulfur on TiO2(110) studied with resonant photoemission. Phys. Rev. B 64, 115418 (2001).
Mattesini, M. et al. High-pressure and high-temperature synthesis of the cubic TiO2 polymorph. Phys. Rev. B 70, 212101 (2004).
Rawat, V., Zakharov, D. N., Stach, E. A. & Sands, T. D. Pseudomorphic stabilization of rocksalt GaN in TiN/GaN multilayers and superlattices. Phys. Rev. B 80, 024114 (2009).
Ohno, T., Sarukawa, K. & Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 26, 1167–1170 (2002).
Losovyj, Y. et al. Optimization of the 3m TGM beamline, at CAMD, for constant initial state spectroscopy. Nucl. Instrum. Meth. A 582, 264–266 (2007).
Hüfner, S. Photoelectron Spectroscopy: Principles and Applications (Springer, 2003).
Acknowledgements
The photoemission data were collected at the Center for Advanced Microstructures and Devices (CAMD) in Baton Rouge, LA, operated by the Louisiana State University (LSU). The authors acknowledge financial support from the US Department of Energy, Office of Basic Energy Sciences (grant no. DE-SC0001508).
Author information
Authors and Affiliations
Contributions
J.T. performed the experiments, analysed the data and wrote the paper. T.L. assisted with the experiments. M.B. directed the research and wrote the paper. All authors read and approved the contents of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 577 kb)
Rights and permissions
About this article
Cite this article
Tao, J., Luttrell, T. & Batzill, M. A two-dimensional phase of TiO2 with a reduced bandgap. Nature Chem 3, 296–300 (2011). https://doi.org/10.1038/nchem.1006
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1006
This article is cited by
-
Modulated transparent conductive zinc oxide films for efficient water splitting
Applied Physics A (2024)
-
Plasmonic core–shell nanoparticles of Ag@TiO2 for photocatalytic degradation of Rhodamine B
Applied Nanoscience (2023)
-
The photocatalytic process in the treatment of polluted water
Chemical Papers (2023)
-
Engineered disorder in CO2 photocatalysis
Nature Communications (2022)
-
Stabilizing photo-induced vacancy defects in MOF matrix for high-performance SERS detection
Nano Research (2022)